Ruthenium Complexes with Protic Ligands: Influence of the Position of OH Groups and π Expansion on Luminescence and Photocytotoxicity
- PMID: 36983054
- PMCID: PMC10053956
- DOI: 10.3390/ijms24065980
Ruthenium Complexes with Protic Ligands: Influence of the Position of OH Groups and π Expansion on Luminescence and Photocytotoxicity
Abstract
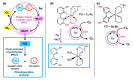
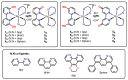
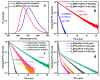
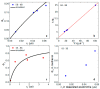
Protic ruthenium complexes using the dihydroxybipyridine (dhbp) ligand combined with a spectator ligand (N,N = bpy, phen, dop, Bphen) have been studied for their potential activity vs. cancer cells and their photophysical luminescent properties. These complexes vary in the extent of π expansion and the use of proximal (6,6'-dhbp) or distal (4,4'-dhbp) hydroxy groups. Eight complexes are studied herein as the acidic (OH bearing) form, [(N,N)2Ru(n,n'-dhbp)]Cl2, or as the doubly deprotonated (O- bearing) form. Thus, the presence of these two protonation states gives 16 complexes that have been isolated and studied. Complex 7A, [(dop)2Ru(4,4'-dhbp)]Cl2, has been recently synthesized and characterized spectroscopically and by X-ray crystallography. The deprotonated forms of three complexes are also reported herein for the first time. The other complexes studied have been synthesized previously. Three complexes are light-activated and exhibit photocytotoxicity. The log(Do/w) values of the complexes are used herein to correlate photocytotoxicity with improved cellular uptake. For Ru complexes 1-4 bearing the 6,6'-dhbp ligand, photoluminescence studies (all in deaerated acetonitrile) have revealed that steric strain leads to photodissociation which tends to reduce photoluminescent lifetimes and quantum yields in both protonation states. For Ru complexes 5-8 bearing the 4,4'-dhbp ligand, the deprotonated Ru complexes (5B-8B) have low photoluminescent lifetimes and quantum yields due to quenching that is proposed to involve the 3LLCT excited state and charge transfer from the [O2-bpy]2- ligand to the N,N spectator ligand. The protonated OH bearing 4,4'-dhbp Ru complexes (5A-8A) have long luminescence lifetimes which increase with increasing π expansion on the N,N spectator ligand. The Bphen complex, 8A, has the longest lifetime of the series at 3.45 μs and a photoluminescence quantum yield of 18.7%. This Ru complex also exhibits the best photocytotoxicity of the series. A long luminescence lifetime is correlated with greater singlet oxygen quantum yields because the triplet excited state is presumably long-lived enough to interact with 3O2 to yield 1O2.
Keywords: anticancer; highly conjugated ligands; light activation; luminescence; protic ligands; ruthenium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fiedler E.C., Hemann M.T. Aiding and Abetting: How the Tumor Microenvironment Protects Cancer from Chemotherapy. Annu. Rev. Cancer Biol. 2019;3:409–428. doi: 10.1146/annurev-cancerbio-030518-055524. - DOI
-
- Monro S., Colón K.L., Yin H., Roque J., Konda P., Gujar S., Thummel R.P., Lilge L., Cameron C.G., McFarland S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019;119:797–828. doi: 10.1021/acs.chemrev.8b00211. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

