Clinical Predictors and Prediction Models for rFVIII-Fc Half Life in Real-World People with Severe Hemophilia A
- PMID: 36983209
- PMCID: PMC10053229
- DOI: 10.3390/jcm12062207
Clinical Predictors and Prediction Models for rFVIII-Fc Half Life in Real-World People with Severe Hemophilia A
Abstract
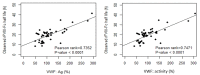
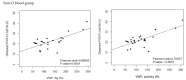
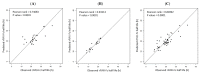
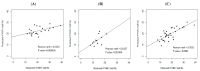
The half life of recombinant factor VIII-Fc (rFVIII-Fc) for people with hemophilia A (PwHA) varies greatly. Understanding the factors influencing the variation and assessment of rFVIII-Fc half life is important for personalized treatment. Eighty-five severe-type PwHA with rFVIII-Fc treatment receiving an evaluation of half life by the Web-Accessible Population Pharmacokinetic (PK) Service-Hemophilia during 2019-2021 were retrospectively enrolled. The 50-patient PK profiles before 2021 were used for analysis and developing prediction models of half life, and the 35-patient PK profiles in 2021 were used for external validation. The patients in the development cohort were aged 8-64, with a median rFVIII-Fc half life of 20.75 h (range, 8.25-41.5 h). By multivariate linear regression analysis, we found two, four, and five predictors of rFVIII-Fc half life for the blood groups non-O, O patients, and overall patients, respectively, including baseline VWF:Ag, BMI, VWF:activity/VWF:Ag ratio, body weight, O blood group, inhibitor history, HCV infection, and hematocrit. The three prediction equations of rFVIII-Fc half life (T) were respectively developed as T for non-O group patients = -0.81 + 0.63 × (BMI, kg/m2) + 6.07 × (baseline VWF:Ag, IU/mL), T for O group patients = -0.68 + 13.30 × (baseline VWF:Ag, IU/mL) + 0.27 × (BW, kg) - 1.17 × (BMI, kg/m2) + 16.02 × (VWF:activity/VWF:Ag ratio), and T for overall patients = -1.76 + 7.24 × (baseline VWF:Ag, IU/mL) - 3.84 × (Inhibitor history) + 2.99 × (HCV infection) - 2.83 × (O blood group) + 0.30 × (Hct, %), which explained 51.97%, 75.17%, and 66.38% of the half life variability, respectively. For external validation, there was a significant correlation between the predicted and observed half lives in the validation cohort. The median half life deviation was +1.53 h, +1.28 h, and +1.79 h for the equations of non-O group, O group, and overall group patients, respectively. In total, eight predictors influencing rFVIII-Fc half life were identified. Prediction equations of rFVIII-Fc half life were developed for the non-O and O blood groups and overall PwHA with a good degree of external validation. The equations could be applied to patients aged 8-64 without the need for PK blood sampling and clinically valuable for personalized therapy.
Keywords: external validation; half life; hemophilia A; pharmacokinetics; prediction model; predictor; rFVIII-Fc.
Conflict of interest statement
The authors state that they have no interests that might be perceived as posing a conflict or bias.
Figures


References
-
- Manco-Johnson M.J., Abshire T.C., Shapiro A.D., Riske B., Hacker M.R., Kilcoyne R., Ingram J.D., Manco-Johnson M.L., Funk S., Jacobson L., et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. - DOI - PubMed
-
- Fischer K., van der Bom J.G., Mauser-Bunschoten E.P., Roosendaal G., Prejs R., de Kleijn P., Grobbee D.E., van den Berg M. The effects of postponing prophylactic treatment on long-term outcome in patients with severe hemophilia. Blood. 2002;99:2337–2341. doi: 10.1182/blood.V99.7.2337. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

