Cell Proliferation, Viability, Differentiation, and Apoptosis of Iron Oxide Labeled Stem Cells Transfected with Lipofectamine Assessed by MRI
- PMID: 36983399
- PMCID: PMC10054380
- DOI: 10.3390/jcm12062395
Cell Proliferation, Viability, Differentiation, and Apoptosis of Iron Oxide Labeled Stem Cells Transfected with Lipofectamine Assessed by MRI
Abstract
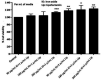
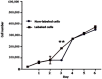
To assess in vitro and in vivo tracking of iron oxide labeled stem cells transfected by lipofectamine using magnetic resonance imaging (MRI), rat dental pulp stem cells (DPSCs) were characterized, labeled with iron oxide nanoparticles, and then transfected with lipofectamine to facilitate the internalization of these nanoparticles. Cell proliferation, viability, differentiation, and apoptosis were investigated. Prussian blue staining and MRI were used to trace transfected labeled cells. DPSCs were a morphologically spindle shape, adherent to culture plates, and positive for adipogenic and osteogenic inductions. They expressed CD73 and CD90 markers and lacked CD34 and CD45. Iron oxide labeling and transfection with lipofectamine in DPSCs had no toxic impact on viability, proliferation, and differentiation, and did not induce any apoptosis. In vitro and in vivo internalization of iron oxide nanoparticles within DPSCs were confirmed by Prussian blue staining and MRI tracking. Prussian blue staining and MRI tracking in the absence of any toxic effects on cell viability, proliferation, differentiation, and apoptosis were safe and accurate to track DPSCs labeled with iron oxide and transfected with lipofectamine. MRI can be a useful imaging modality when treatment outcome is targeted.
Keywords: MRI; dental pulp stem cells; iron oxide nanoparticles; lipofectamine; tracking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
MRI-Tracking of Dental Pulp Stem Cells In Vitro and In Vivo Using Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles.J Clin Med. 2019 Sep 9;8(9):1418. doi: 10.3390/jcm8091418. J Clin Med. 2019. PMID: 31505807 Free PMC article.
-
Transfection of neuroprogenitor cells with iron nanoparticles for magnetic resonance imaging tracking: cell viability, differentiation, and intracellular localization.Mol Imaging Biol. 2005 Jul-Aug;7(4):286-95. doi: 10.1007/s11307-005-0008-1. Mol Imaging Biol. 2005. PMID: 16080022
-
[In vivo magnetic resonance imaging tracking of transplanted adipose-derived stem cells labeled with superparamagnetic iron oxide in rat hearts].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009 Apr;31(2):187-91. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009. PMID: 19507598 Chinese.
-
In vivo tracking of stem cells in brain and spinal cord injury.Prog Brain Res. 2007;161:367-83. doi: 10.1016/S0079-6123(06)61026-1. Prog Brain Res. 2007. PMID: 17618991 Review.
-
Application of iron oxide nanoparticles in the diagnosis and treatment of leukemia.Front Pharmacol. 2023 Mar 30;14:1177068. doi: 10.3389/fphar.2023.1177068. eCollection 2023. Front Pharmacol. 2023. PMID: 37063276 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cell-based Scaffolds in Regenerative Medicine of Dental Diseases.Stem Cell Rev Rep. 2024 Apr;20(3):688-721. doi: 10.1007/s12015-024-10687-6. Epub 2024 Feb 3. Stem Cell Rev Rep. 2024. PMID: 38308730 Review.
-
Bone marrow stem cells with or without superparamagnetic iron oxide nanoparticles as a magnetic targeting tool: Which is better in regeneration of neurolysed facial nerve? An experimental study.Heliyon. 2024 Feb 17;10(4):e26675. doi: 10.1016/j.heliyon.2024.e26675. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38434051 Free PMC article.
-
Berberine Inhibits Ferroptosis and Stabilizes Atherosclerotic Plaque through NRF2/SLC7A11/GPX4 Pathway.Chin J Integr Med. 2024 Oct;30(10):906-916. doi: 10.1007/s11655-024-3666-z. Epub 2024 Aug 21. Chin J Integr Med. 2024. PMID: 39167283
-
Magnetic nanoparticles influence the biological function of mesenchymal stem cells.Sci Rep. 2025 Jul 30;15(1):27862. doi: 10.1038/s41598-025-13083-3. Sci Rep. 2025. PMID: 40739120 Free PMC article.
-
The impact of acemannan, an extracted product from Aloe vera, on proliferation of dental pulp stem cells and healing of mandibular defects in rabbits.Am J Stem Cells. 2024 Apr 25;13(2):75-86. doi: 10.62347/UAFC3719. eCollection 2024. Am J Stem Cells. 2024. PMID: 38765804 Free PMC article.
References
-
- Mehrabani D., Arshi S., Sadeghi L., Khodabandeh Z., Zare S., Rabiee M. The ameliorating effect of adipose tissue stem cells on liver function in experimental rats with liver fibrosis. Int. J. Nutr. Sci. 2022;7:225–232.
-
- Mehrabani D., Rabiee M., Tamadon A., Zare S., Razeghian Jahromi I., Dianatpour M., Khodabandeh Z. The growth kinetic, differentiation properties, karyotyping, and characterization of adipose tissue-derived stem cells in hamster. Comp. Clin. Pathol. 2016;25:1017–1022. doi: 10.1007/s00580-016-2300-7. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

