Epidemiology of Immune-Mediated Glomerulopathies before and after SARS-CoV-2 Vaccination: A Tertiary Referral Hospital Experience
- PMID: 36983419
- PMCID: PMC10056116
- DOI: 10.3390/jcm12062420
Epidemiology of Immune-Mediated Glomerulopathies before and after SARS-CoV-2 Vaccination: A Tertiary Referral Hospital Experience
Abstract
Background: Vaccination is a known trigger for the appearance of immune-mediated glomerulopathies (IMG). The appearance of IMG after SARS-CoV-2 vaccination with suspected causality has been described. Our aim is to analyze the incidence of IMG flares before and after SARS-CoV-2 vaccination in our center.
Methods: All persons with native kidney biopsy (KB) from January 2019 to March 2022 in our center were included in the study. We compared the incidence of IMG before and after the start of vaccination. We also collected information about whether the patients had received a SARS-CoV-2 vaccine or have suffered from COVID in the six weeks before the IMG. We also evaluated the analytical characteristics of the outbreaks.
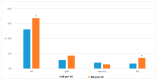
Results: A total of 386 KB were studied. Of them, 86/218 (39.4%) were IMG performed pre- and 85/168 (50.6%) post-SV (029). The incidence of idiopathic nephrotic syndrome (INS), studied separately, was also significantly increased post-vaccination (n = 18 (10.7%)) compared to pre-vaccination (n = 11 (5%)) (p = 0.036). There were no differences in the incidence of vasculitis or IgA nephropathy. Up to 17 (20%) flares occurred 6 weeks before SARS-CoV-2 vaccination and only 2 (2.4%) within the first 6 weeks after SARS-CoV-2 infection. Within those 17 flares, the most common diagnosis was IgAN (n = 5 (29.4%)); a total of 14 (82.4%) received an mRNA vaccine and 9 (52.9%) took place after the 1st vaccine dose. There were 13 cases of minimal change disease (MCD) with debut/recurrence pre-SV and 20 MCD with debut/recurrence post-SV (p = 0.002).
Conclusions: The incidence of IMG, INS and MCD flares in our center increased significantly after SARS-CoV-2 vaccination. Importantly, 20% of IMG flares took place within the first 6 weeks after receiving a vaccine dose, with the first dose being the riskiest one and IgAN the most frequent diagnosis.
Keywords: SARS-CoV-2; flare; immune-mediated glomerulopathy; vaccine.
Conflict of interest statement
M.L.-M. received support for attending meetings and/or travel from Sanofi and AstraZeneca. S.B. received consulting fees and payment for lectures, presentations, speakers, bureaus, manuscript writing or educational events, and for participation on a data safety monitoring board or on a advisory board from AstraZeneca and Mundipharma. A.V. received grants or contracts from Instituto Carlos III (ISCIII) and Fundación Alfonso Martín Escudero, support for attending meetings and/or travel from Mundipharma, Sanofi, and Novo Nordisk. N.R.T. received grants for participation on a data safety monitoring board or advisory board from Alexion. M.J.S. received grants or contracts from Boehringer, ISCIII, and Marató TV3; honoraria for lectures from NovoNordisk, Jansen, Boehringer, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor, ICU Medical, Fresenius, and Travere Therapeutics; support for attending meetings from Travere; and participated on a data safety, monitoring board or advisory board from NovoNordisk, Jansen, Boehringer, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor, ICU Medical, Bayer, GE Healthcare, and Travere Therapeutics. M.J.S. has the following leadership or fiduciary roles: SEC Board member, SEN board member, ex-ERA board member, ex-ASN Board News, ex-ERA-EDTA SAB, ex-council member of ERA, elected EIC CKJ. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., Wiedmann M., Aamodt A.-H., Skattør T.H., Tjønnfjord G.E., et al. Thrombosis and Thrombocytopenia after ChAdOx1 NCoV-19 Vaccination. N. Engl. J. Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. - DOI - PMC - PubMed
-
- Oster M.E., Shay D.K., Su J.R., Gee J., Creech C.B., Broder K.R., Edwards K., Soslow J.H., Dendy J.M., Schlaudecker E., et al. Myocarditis Cases Reported after MRNA-Based COVID-19 Vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331. doi: 10.1001/jama.2021.24110. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous