The Optimal Management of Inflammatory Bowel Disease in Patients with Cancer
- PMID: 36983432
- PMCID: PMC10056442
- DOI: 10.3390/jcm12062432
The Optimal Management of Inflammatory Bowel Disease in Patients with Cancer
Abstract
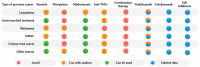
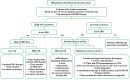
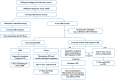
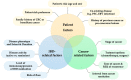
Patients with inflammatory bowel disease (IBD) have an increased risk of cancer secondary to chronic inflammation and long-term use of immunosuppressive therapy. With the aging IBD population, the prevalence of cancer in IBD patients is increasing. As a result, there is increasing concern about the impact of IBD therapy on cancer risk and survival, as well as the effects of cancer therapies on the disease course of IBD. Managing IBD in patients with current or previous cancer is challenging since clinical guidelines are based mainly on expert consensus. Evidence is rare and mainly available from registries or observational studies. In contrast, excluding patients with previous/or active cancer from clinical trials and short-term follow-up can lead to an underestimation of the cancer or cancer recurrence risk of approved medications. The present narrative review aims to summarize the current evidence and provide practical guidance on the management of IBD patients with cancer.
Keywords: Crohn’s disease; anti-tumor necrosis factors; biologic; cancer; inflammatory bowel disease; risk; thiopurine; ulcerative colitis; ustekinumab; vedolizumab.
Conflict of interest statement
P.W. has been a speaker and/or advisory board member for Takeda, Pfizer, Janssen, Ferring, A. Menerini, Sandoz, and MSD. T.B. has been a speaker or advisory board member for Takeda, Janssen, Abbvie, Merck, Pfizer, Pendopharm, Ferring, Shire, Sandoz, B.M.S., Roche, Fresenius Kabi, and Viatris. P.L.L. has been a speaker and/or advisory board member for AbbVie, Arena, Falk Pharma GmbH, Ferring, Genetech, Janssen, Kyowa Hakko Kirin Pharma, Mitsubishi Tanabe Pharma Corporation, MSD, Pfizer, Roche, Shire, Takeda, and Tillots, and has received unrestricted research grants from AbbVie, MSD, and Pfizer. P.T. and R.A.J. declared no conflict of interest.
Figures

Similar articles
-
The Impact of Inflammatory Bowel Disease in Canada 2018: Direct Costs and Health Services Utilization.J Can Assoc Gastroenterol. 2019 Feb;2(Suppl 1):S17-S33. doi: 10.1093/jcag/gwy055. Epub 2018 Nov 2. J Can Assoc Gastroenterol. 2019. PMID: 31294382 Free PMC article.
-
The Impact of Inflammatory Bowel Disease in Canada 2018: Children and Adolescents with IBD.J Can Assoc Gastroenterol. 2019 Feb;2(Suppl 1):S49-S67. doi: 10.1093/jcag/gwy056. Epub 2018 Nov 2. J Can Assoc Gastroenterol. 2019. PMID: 31294385 Free PMC article.
-
Therapeutic Management of Adults with Inflammatory Bowel Disease and Malignancies: A Clinical Challenge.Cancers (Basel). 2023 Jan 16;15(2):542. doi: 10.3390/cancers15020542. Cancers (Basel). 2023. PMID: 36672491 Free PMC article. Review.
-
The Impact of Inflammatory Bowel Disease in Canada 2018: Extra-intestinal Diseases in IBD.J Can Assoc Gastroenterol. 2019 Feb;2(Suppl 1):S73-S80. doi: 10.1093/jcag/gwy053. Epub 2018 Nov 2. J Can Assoc Gastroenterol. 2019. PMID: 31294387 Free PMC article.
-
Biologics in inflammatory bowel disease: what are the data?United European Gastroenterol J. 2015 Oct;3(5):419-28. doi: 10.1177/2050640615590302. United European Gastroenterol J. 2015. PMID: 26535119 Free PMC article. Review.
Cited by
-
Inflammatory Bowel Disease and Colorectal Cancer.Cancers (Basel). 2024 Aug 23;16(17):2943. doi: 10.3390/cancers16172943. Cancers (Basel). 2024. PMID: 39272800 Free PMC article. Review.
-
Does Elderly-Onset Inflammatory Bowel Disease Increase Risk of Colorectal Cancer? A Systematic Review and Meta-Analysis.J Clin Med. 2023 Dec 27;13(1):148. doi: 10.3390/jcm13010148. J Clin Med. 2023. PMID: 38202155 Free PMC article. Review.
-
Advancements in Inflammatory Bowel Disease: A Narrative Review of Diagnostics, Management, Epidemiology, Prevalence, Patient Outcomes, Quality of Life, and Clinical Presentation.Cureus. 2023 Jun 28;15(6):e41120. doi: 10.7759/cureus.41120. eCollection 2023 Jun. Cureus. 2023. PMID: 37519622 Free PMC article. Review.
-
Interaction Effect of Psoriasis and Cancer on the Risk of All-Cause Mortality: A Prospective Cohort Study of NHANES Data.Indian J Dermatol. 2024 Jul-Aug;69(4):317-327. doi: 10.4103/ijd.ijd_1095_23. Epub 2024 Aug 19. Indian J Dermatol. 2024. PMID: 39296686 Free PMC article.
References
-
- Kappelman M.D., Farkas D.K., Long M.D., Erichsen R., Sandler R.S., Sørensen H.T., Baron J.A. Risk of Cancer in Patients with Inflammatory Bowel Diseases: A Nationwide Population-Based Cohort Study with 30 Years of Follow-up Evaluation. Clin. Gastroenterol. Hepatol. 2014;12:265–273.e1. doi: 10.1016/j.cgh.2013.03.034. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

