What Is New in Pulmonary Mucormycosis?
- PMID: 36983475
- PMCID: PMC10057210
- DOI: 10.3390/jof9030307
What Is New in Pulmonary Mucormycosis?
Abstract
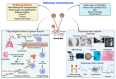
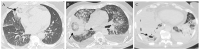
Mucormycosis is a rare but life-threatening fungal infection due to molds of the order Mucorales. The incidence has been increasing over recent decades. Worldwide, pulmonary mucormycosis (PM) presents in the lungs, which are the third main location for the infection after the rhino-orbito-cerebral (ROC) areas and the skin. The main risk factors for PM include hematological malignancies and solid organ transplantation, whereas ROC infections classically are classically favored by diabetes mellitus. The differences between the ROC and pulmonary locations are possibly explained by the activation of different mammalian receptors-GRP78 in nasal epithelial cells and integrin β1 in alveolar epithelial cells-in response to Mucorales. Alveolar macrophages and neutrophils play a key role in the host defense against Mucorales. The diagnosis of PM relies on CT scans, cultures, PCR tests, and histology. The reversed halo sign is an early, but very suggestive, sign of PM in neutropenic patients. Recently, the serum PCR test showed a very encouraging performance for the diagnosis and follow-up of mucormycosis. Liposomal amphotericin B is the drug of choice for first-line therapy, together with correction of underlying disease and surgery when feasible. After a stable or partial response, the step-down treatment includes oral isavuconazole or posaconazole delayed release tablets until a complete response is achieved. Secondary prophylaxis should be discussed when there is any risk of relapse, such as the persistence of neutropenia or the prolonged use of high-dose immunosuppressive therapy. Despite these novelties, the mortality rate from PM remains higher than 50%. Therefore, future research must define the place for combination therapy and adjunctive treatments, while the development of new treatments is necessary.
Keywords: Epidemiology; Mucorales; pulmonary mucormycosis; review; treatment.
Conflict of interest statement
F.D. has received honoraria for conferences from Gilead and Pfizer, outside the submitted work. C.M. declares personal fees from Gilead. F.L. (Lamoth) has received research funding from Gilead, MSD, Pfizer and Novartis, and honoraria for advisory boards or conferences from Gilead, MSD, Pfizer and Mundipharma. All contracts were made with and fees paid to his institution (CHUV). T.C. has participated in advisory boards or consulted for MSD Merck & Dohme, Menarini, Shionogi, BD, CSL Behring, Gentian, Cytosorbent, ThermoFisher Scientific, GE Healthcare, Volition for projects unrelated to the submitted work and on data safety monitoring boards for Cidara, Novartis and Lymphatica. All contracts were made with and fees paid to his institution (CHUV). F.L. (Lanternier) declares personal fees from Gilead, F2G and Pfizer. O.L. has consulted for Gilead. All other authors declare no conflict of interest.
Figures



References
-
- Bretagne S., Sitbon K., Desnos-Ollivier M., Garcia-Hermoso D., Letscher-Bru V., Cassaing S., Millon L., Morio F., Gangneux J.-P., Hasseine L., et al. Active Surveillance Program to Increase Awareness on Invasive Fungal Diseases: The French RESSIF Network (2012 to 2018) mBio. 2022;13:e0092022. doi: 10.1128/mbio.00920-22. - DOI - PMC - PubMed
-
- Guinea J., Escribano P., Vena A., Muñoz P., Martínez-Jiménez M.D.C., Padilla B., Bouza E. Increasing Incidence of Mucormycosis in a Large Spanish Hospital from 2007 to 2015: Epidemiology and Microbiological Characterization of the Isolates. PLoS ONE. 2017;12:e0179136. doi: 10.1371/journal.pone.0179136. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

