Anti-Biofilm Activity of Phenyllactic Acid against Clinical Isolates of Fluconazole-Resistant Candida albicans
- PMID: 36983523
- PMCID: PMC10054014
- DOI: 10.3390/jof9030355
Anti-Biofilm Activity of Phenyllactic Acid against Clinical Isolates of Fluconazole-Resistant Candida albicans
Abstract
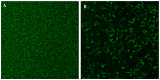
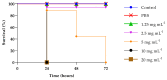
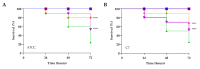
Commonly found colonizing the human microbiota, Candida albicans is a microorganism known for its ability to cause infections, mainly in the vulvovaginal region, and is responsible for 85% to 90% of vulvovaginal candidiasis (VVC) cases. The development of drug resistance in C. albicans isolates after long-term therapy with fluconazole is an important complication to solve and new therapeutic strategies are required to target this organism and its pathogenicity. In the present study, phenyllactic acid (PLA) an important broad-spectrum antimicrobial compound was investigated for its antifungal and antivirulence activities against clinical isolates of C. albicans. Previously characterized strains of C. albicans isolates from women with VVC and C. albicans ATCC90028 were used to evaluate the antimicrobial and time dependent killing assay activity of PLA showing a MIC 7.5 mg mL-1 and a complete reduction of viable Candida cells detected by killing kinetics after 4 h of treatment with PLA. Additionally, PLA significantly reduced the biomass and the metabolic activity of C. albicans biofilms and impaired biofilm formation also with changes in ERG11, ALS3, and HWP1 genes expression as detected by qPCR. PLA eradicated pre-formed biofilms as showed also with confocal laser scanning microscopy (CLSM) observations. Furthermore, the compound prolonged the survival rate of Galleria mellonella infected by C. albicans isolates. These results indicate that PLA is a promising candidate as novel and safe antifungal agents for the treatment of vulvovaginal candidiasis.
Keywords: Candida albicans; Galleria mellonella; biofilm; fluconazole resistance; phenyllactic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Calvet G., Aguiar R.S., Melo A.S., Sampaio S.A., De Filippis I., Fabri A., Araujo E.S., de Sequeira P.C., de Mendonça M.C., de Oliveira L. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016;16:653–660. doi: 10.1016/S1473-3099(16)00095-5. - DOI - PubMed
-
- Anh D.N., Hung D.N., Tien T.V., Dinh V.N., Son V.T., Luong N.V., Van N.T., Quynh N.T.N., Van Tuan N., Tuan L.Q., et al. Prevalence, species distribution and antifungal susceptibility of Candida albicans causing vaginal discharge among symptomatic non-pregnant women of reproductive age at a tertiary care hospital, Vietnam. BMC Infect. Dis. 2021;21:523. doi: 10.1186/s12879-021-06192-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

