Silver(I) 1,10-Phenanthroline Complexes Are Active against Fonsecaea pedrosoi Viability and Negatively Modulate Its Potential Virulence Attributes
- PMID: 36983524
- PMCID: PMC10057124
- DOI: 10.3390/jof9030356
Silver(I) 1,10-Phenanthroline Complexes Are Active against Fonsecaea pedrosoi Viability and Negatively Modulate Its Potential Virulence Attributes
Abstract
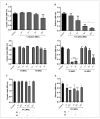
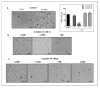
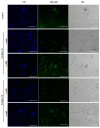
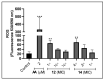
The genus Fonsecaea is one of the etiological agents of chromoblastomycosis (CBM), a chronic subcutaneous disease that is difficult to treat. This work aimed to evaluate the effects of copper(II), manganese(II) and silver(I) complexes coordinated with 1,10-phenanthroline (phen)/1,10-phenanthroline-5,6-dione (phendione) on Fonsecaea spp. Our results revealed that most of these complexes were able to inhibit F. pedrosoi, F. monophora and F. nubica conidial viability with minimum inhibitory concentration (MIC) values ranging from 0.6 to 100 µM. The most effective complexes against F. pedrosoi planktonic conidial cells, the main etiologic agent of CBM, were [Ag(phen)2]ClO4 and [Ag2(3,6,9-tdda)(phen)4].EtOH, (tdda: 3,6,9-trioxaundecanedioate), displaying MIC values equal to 1.2 and 0.6 µM, respectively. These complexes were effective in reducing the viability of F. pedrosoi biofilm formation and maturation. Silver(I)-tdda-phen, combined with itraconazole, reduced the viability and extracellular matrix during F. pedrosoi biofilm development. Moreover, both silver(I) complexes inhibited either metallo- or aspartic-type peptidase activities of F. pedrosoi as well as its conidia into mycelia transformation and melanin production. In addition, the complexes induced the production of intracellular reactive oxygen species in F. pedrosoi. Taken together, our data corroborate the antifungal action of metal-phen complexes, showing they represent a therapeutic option for fungal infections, including CBM.
Keywords: antifungal activity; chromoblastomycosis; metal-based drugs; virulence factors.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- WHO . Tenth Report of the Strategic and Technical Advisory Group for Neglected Tropical Diseases. World Health Organization; Geneva, Switzerland: 2017.
-
- Viganor L., Howe O., Mccarron P., Mccann M., Devereux M. The antibacterial activity of metal complexes containing 1,10- phenanthroline: Potential as alternative therapeutics in the era of antibiotic resistance. Curr. Top. Med. Chem. 2017;17:1280–1302. doi: 10.2174/1568026616666161003143333. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

