Melanoma and Nevi Subtype Histopathological Characterization with Optical Coherence Tomography
- PMID: 36983781
- PMCID: PMC10058073
- DOI: 10.3390/life13030625
Melanoma and Nevi Subtype Histopathological Characterization with Optical Coherence Tomography
Abstract
Background: Melanoma incidence has continued to rise in the latest decades, and the forecast is not optimistic. Non-invasive diagnostic imaging techniques such as optical coherence tomography (OCT) are largely studied; however, there is still no agreement on its use for the diagnosis of melanoma. For dermatologists, the differentiation of non-invasive (junctional nevus, compound nevus, intradermal nevus, and melanoma in-situ) versus invasive (superficial spreading melanoma and nodular melanoma) lesions is the key issue in their daily routine.
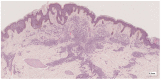
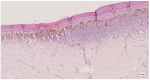
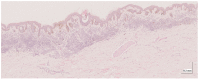
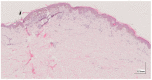
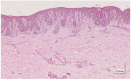
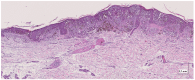
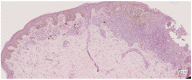
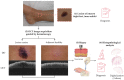
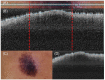
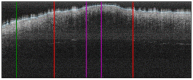
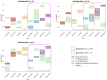
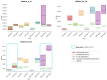
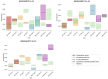
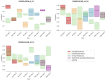
Methods: This work performs a comparative analysis of OCT images using haematoxylin-eosin (HE) and anatomopathological features identified by a pathologist. Then, optical and textural properties are extracted from OCT images with the aim to identify subtle features that could potentially maximize the usefulness of the imaging technique in the identification of the lesion's potential invasiveness.
Results: Preliminary features reveal differences discriminating melanoma in-situ from superficial spreading melanoma and also between melanoma and nevus subtypes that pose a promising baseline for further research.
Conclusions: Answering the final goal of diagnosing non-invasive versus invasive lesions with OCT does not seem feasible in the short term, but the obtained results demonstrate a step forward to achieve this.
Keywords: CADx; HE; OCT; histopathology; melanoma; optical biopsy; optical properties; skin cancer; textural properties.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






References
-
- Skin Cancer Statistics. World Cancer Research Fund International. [(accessed on 2 August 2022)]. Available online: https://www.wcrf.org/cancer-trends/skin-cancer-statistics/
-
- Ferrante di Ruffano L., Dinnes J., Deeks J.J., Chuchu N., Bayliss S.E., Davenport C., Takwoingi Y., Godfrey K., O’sullivan C., Matin R.N., et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018;2018:CD013189. doi: 10.1002/14651858.CD013189. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

