Target Trial Emulation Using Hospital-Based Observational Data: Demonstration and Application in COVID-19
- PMID: 36983933
- PMCID: PMC10053871
- DOI: 10.3390/life13030777
Target Trial Emulation Using Hospital-Based Observational Data: Demonstration and Application in COVID-19
Abstract
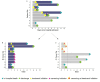
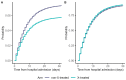
Methodological biases are common in observational studies evaluating treatment effectiveness. The objective of this study is to emulate a target trial in a competing risks setting using hospital-based observational data. We extend established methodology accounting for immortal time bias and time-fixed confounding biases to a setting where no survival information beyond hospital discharge is available: a condition common to coronavirus disease 2019 (COVID-19) research data. This exemplary study includes a cohort of 618 hospitalized patients with COVID-19. We describe methodological opportunities and challenges that cannot be overcome applying traditional statistical methods. We demonstrate the practical implementation of this trial emulation approach via clone-censor-weight techniques. We undertake a competing risk analysis, reporting the cause-specific cumulative hazards and cumulative incidence probabilities. Our analysis demonstrates that a target trial emulation framework can be extended to account for competing risks in COVID-19 hospital studies. In our analysis, we avoid immortal time bias, time-fixed confounding bias, and competing risks bias simultaneously. Choosing the length of the grace period is justified from a clinical perspective and has an important advantage in ensuring reliable results. This extended trial emulation with the competing risk analysis enables an unbiased estimation of treatment effects, along with the ability to interpret the effectiveness of treatment on all clinically important outcomes.
Keywords: COVID-19; competing events; methodology; observational data; target trial emulation.
Conflict of interest statement
D.H. was supported by Innovative Medicines Initiative Joint Undertaking under grant agreement n 115737 resources of which are composed of financial contribution from the European Union Seventh Framework Programme (FP7/2007-2013) and EFPIA companies in kind contribution. H.R.M. was supported by the Beatriu de Pinós post-doctoral programme from the Office of the Secretary of Universities and Research from the Ministry of Business and Knowledge of the Government of Catalonia programme (#2020 BP 00261). M.M. received a grant from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 712949 (TECNIOspring PLUS) and from the Agency for Business Competitiveness of the Government of Catalonia (TECSPR18-1–0017). All other authors report no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

