In Vitro Effects of Photon Beam and Carbon Ion Radiotherapy on the Perineural Invasion of Two Cell Lines of Neurotropic Tumours
- PMID: 36983949
- PMCID: PMC10056732
- DOI: 10.3390/life13030794
In Vitro Effects of Photon Beam and Carbon Ion Radiotherapy on the Perineural Invasion of Two Cell Lines of Neurotropic Tumours
Abstract
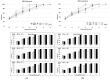
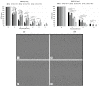
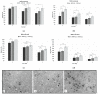
Primary mucosal melanoma (PMM) and pancreatic ductal adenocarcinoma (PDAC) are two aggressive malignancies, characterized by intrinsic radio-chemoresistance and neurotropism, a histological feature resulting in frequent perineural invasion (PNI), supported by neurotrophic factors secreted in the tumour microenvironment (TME), such as neurotrophin-3 (NT-3). Carbon-ion radiotherapy (CIRT) could represent an effective option in unresectable PMM and PDAC. Only a few data about the effects of CIRT on PNI in relation to NT-3 are available in the literature, despite the numerous pieces of evidence revealing the peculiar effects of this type of radiation on tumour cell migration. This in vitro study investigated for the first time the response of PMM and PDAC cells to NT-3 and evaluated the effects of conventional photon beam radiotherapy (XRT) and CIRT on cell viability, proliferation, and migration. Our results demonstrated the greater capacity of C-ions to generally decrease cell viability, proliferation, and migration, while the addition of NT-3 after both types of irradiation determined an increase in these features, maintaining a dose-dependent trend and acting more effectively as a chemoattractant than inductor in the case of migration.
Keywords: NT-3; carbon ions; hadrontherapy; migration; neurotropism; perineural invasion; radiobiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

