Detection of Lymphatic Vessels in the Superficial Fascia of the Abdomen
- PMID: 36983991
- PMCID: PMC10058564
- DOI: 10.3390/life13030836
Detection of Lymphatic Vessels in the Superficial Fascia of the Abdomen
Abstract
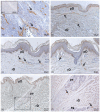
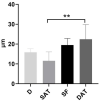
Recently, the superficial fascia has been recognized as a specific anatomical structure between the two adipose layers-the superficial adipose tissue (SAT) and the deep adipose tissue (DAT). The evaluation of specific characteristics of cells, fibers, blood circulation, and innervation has shown that the superficial fascia has a clear and distinct anatomical identity, but knowledge about lymphatic vessels in relation to the superficial fascia has not been described. The aim of this study was to evaluate the presence of lymphatic vessels in the hypodermis, with a specific focus on the superficial fascia and in relation to the layered subdivision of the subcutaneous tissue into SAT and DAT. Tissue specimens were harvested from three adult volunteer patients during abdominoplasty and stained with D2-40 antibody for the lymphatic endothelium. In the papillary dermis, a huge presence of lymphatic vessels was highlighted, parallel to the skin surface and embedded in the loose connective tissue. In the superficial adipose tissue, thin lymphatic vessels (mean diameter of 11.6 ± 7.71 µm) were found, close to the fibrous septa connecting the dermis to the deeper layers. The deep adipose tissue showed a comparable overall content of lymphatic vessels with respect to the superficial layer; they followed the blood vessel and had a larger diameter. In the superficial fascia, the lymphatic vessels showed higher density and a larger diameter, in both the longitudinal and transverse directions along the fibers, as well as vessels that intertwined with one another, forming a rich network of vessels. This study demonstrated a different distribution of the lymphatic vessels in the various subcutaneous layers, especially in the superficial fascia, and the demonstration of the variable gauge of the vessels leads us to believe that they play different functional roles in the collection and transport of interstitial fluid-important factors in various surgical and rehabilitation fields.
Keywords: hypodermis; immunohistochemistry; lymphatic system; lymphatics vessels; reconstructive surgery; subcutaneous tissue; superficial fascia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Macchi V., Tiengo C., Porzionato A., Stecco C., Galli S., Vigato E., Azzena B., Parenti A., De Caro R. Anatomo-radiological study of the superficial musculo-aponeurotic system of the face. Ital. J. Anat. Embryol. 2007;112:247–253. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

