Anomalous Properties of Cyclodextrins and Their Complexes in Aqueous Solutions
- PMID: 36984102
- PMCID: PMC10051767
- DOI: 10.3390/ma16062223
Anomalous Properties of Cyclodextrins and Their Complexes in Aqueous Solutions
Abstract
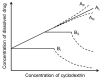
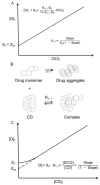
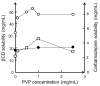
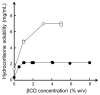
Cyclodextrins (CDs) are cyclic oligosaccharides that emerged as industrial excipients in the early 1970s and are currently found in at least 130 marketed pharmaceutical products, in addition to numerous other consumer products. Although CDs have been the subject of close to 100,000 publications since their discovery, and although their structure and properties appear to be trivial, CDs are constantly surprising investigators by their unique physicochemical properties. In aqueous solutions, CDs are solubilizing complexing agents of poorly soluble drugs while they can also act as organic cosolvents like ethanol. CDs and their complexes self-assemble in aqueous solutions to form both nano- and microparticles. The nanoparticles have diameters that are well below the wavelength of visible light; thus, the solutions appear to be clear. However, the nanoparticles can result in erroneous conclusions and misinterpretations of experimental results. CDs can act as penetration enhancers, increasing drug permeation through lipophilic membranes, but they do so without affecting the membrane barrier. This review is an account of some of the unexpected results the authors have encountered during their studies of CDs as pharmaceutical excipients.
Keywords: aggregation; cyclodextrins; microparticles; nanoparticles; properties; supersaturated solutions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fenyvesi E., Szente L. Cyclodextrin in starchy foods. Acta Aliment. 2021;50:417–432. doi: 10.1556/066.2021.00051. - DOI
-
- Puská I., Szent L., Szőcs L., Fenyvesi É. Recent list of cyclodextrin-containing drug products. Period. Polytech. Chem. Eng. 2023;67:11–17. doi: 10.3311/PPch.21222. - DOI
-
- Poulson B.G., Alsulami Q.A., Sharfalddin A., El Agammy E.F., Mouffouk F., Emwas A.-H., Jaremko L., Jaremko M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides. 2022;3:1–31. doi: 10.3390/polysaccharides3010001. - DOI
Publication types
LinkOut - more resources
Full Text Sources

