Effect of Plasma Argon Pretreatment on the Surface Properties of AZ31 Magnesium Alloy
- PMID: 36984207
- PMCID: PMC10057725
- DOI: 10.3390/ma16062327
Effect of Plasma Argon Pretreatment on the Surface Properties of AZ31 Magnesium Alloy
Abstract
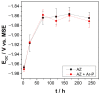
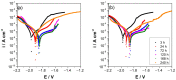
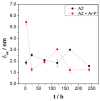
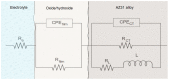
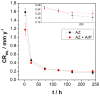
Climate change has evidenced the need to reduce carbon dioxide emissions into the atmosphere, and so for transport applications, lighter weight alloys have been studied, such as magnesium alloys. However, they are susceptible to corrosion; therefore, surface treatments have been extensively studied. In this work, the influence of argon plasma pretreatment on the surface properties of an AZ31 magnesium alloy focus on the enhancement of the reactivity of the surface, which was examined by surface analysis techniques, electrochemical techniques, and gravimetric measurements. The samples were polished and exposed to argon plasma for two minutes in order to activate the surface. Contact angle measurements revealed higher surface energy after applying the pretreatment, and atomic force microscopy showed a roughness increase, while X-Ray photoelectron spectroscopy showed a chemical change on the surface, where after pretreatment the oxygen species increased. Electrochemical measurements showed that surface pretreatment does not affect the corrosion mechanism of the alloy, while electrochemical impedance spectroscopy reveals an increase in the original thickness of the surface film. This increase is likely associated with the high reactivity that the plasma pretreatment confers to the surface of the AZ31 alloy, affecting the extent of oxide formation and, consequently, the increase in its protection capacity. The weight loss measurements support the effect of the plasma pretreatment on the oxide thickness since the corrosion rate of the pretreated AZ31 specimens was lower than that of those that did not receive the surface pretreatment.
Keywords: AZ31 alloy; argon plasma; corrosion; magnesium alloy; surface treatment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Esmaily M., Svensson J.E., Fajardo S., Birbilis N., Frankel G.S., Virtanen S., Arrabal R., Thomas S., Johansson L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017;89:92–193. doi: 10.1016/j.pmatsci.2017.04.011. - DOI
-
- Leleu S., Rives B., Causse N., Pébère N. Corrosion Rate Determination of Rare-Earth Mg Alloys in a Na2SO4 Solution by Electrochemical Measurements and Inductive Coupled Plasma-Optical Emission Spectroscopy. J. Magnes. Alloys. 2019;7:47–57. doi: 10.1016/j.jma.2018.12.002. - DOI
-
- Tiyyagura H.R., Puliyalil H., Filipič G., Kumar K.C., Pottathara Y.B., Rudolf R., Fuchs-Godec R., Mohan M.K., Cvelbar U. Corrosion Studies of Plasma Modified Magnesium Alloy in Simulated Body Fluid (SBF) Solutions. Surf. Coat. Technol. 2020;385:125434. doi: 10.1016/j.surfcoat.2020.125434. - DOI
-
- Yang J., Cui F.Z., Lee I.S., Wang X. Plasma Surface Modification of Magnesium Alloy for Biomedical Application. Surf. Coat. Technol. 2010;205((Suppl. S1)):S182–S187. doi: 10.1016/j.surfcoat.2010.07.045. - DOI
-
- Gray J.E., Luan B. Protective Coatings on Magnesium and Its Alloys—A Critical Review. J. Alloys Compd. 2002;336:88–113. doi: 10.1016/S0925-8388(01)01899-0. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

