Cu3As: Uncommon Crystallographic Features, Low-Temperature Phase Transitions, Thermodynamic and Physical Properties
- PMID: 36984382
- PMCID: PMC10051385
- DOI: 10.3390/ma16062501
Cu3As: Uncommon Crystallographic Features, Low-Temperature Phase Transitions, Thermodynamic and Physical Properties
Abstract
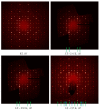
The formation and crystal structure of the binary Cu3As phase have been re-investigated. Some physical properties were then measured on both single crystal and polycrystalline bulk. Cu3As melts congruently at 835 °C. At room temperature (RT), this compound has been found to crystallize in the hexagonal Cu3P prototype (hP24, P63cm) with lattice parameters: a = 7.1393(1) Å and c = 7.3113(1) Å, rather than in the anti HoH3-type (hP24, P-3c1) as indicated in literature. A small compositional range of 74.0-75.5 at.% Cu (26.0-24.5 at.% As) was found for samples synthesized at 300 and 400 °C; a corresponding slight understoichiometry is found in one out of the four Cu atomic sites, leading to the final refined composition Cu2.882(1)As. The present results disprove a change in the crystal structure above RT actually reported in the phase diagram (from γ' to γ on heating). Instead, below RT, at T = 243 K (-30 °C), a first-order structural transition to a trigonal low-temperature superstructure, LT-Cu3-xAs (hP72, P-3c1) has been found. The LT polymorph is metrically related to the RT one, having the c lattice parameter three times larger: a = 7.110(2) Å and c = 21.879(4) Å. Both the high- and low-temperature polymorphs are characterized by the presence of a tridimensional (3D) uncommon and rigid Cu sublattice of the lonsdaleite type (Cu atoms tetrahedrally bonded), which remains almost unaffected by the structural change(s), and characteristic layers of triangular 'Cu3As'-units (each hosting one As atom at the center, interconnected each other by sharing the three vertices). The first-order transition is then followed by an additional structural change when lowering the temperature, which induces doubling of also the lattice parameter a. Differential scanning calorimetry nicely detects the first low-temperature structural change occurring at T = 243 K, with an associated enthalpy difference, ΔH(TR), of approximately 2 J/g (0.53 kJ/mol). Low-temperature electrical resistivity shows a typical metallic behavior; clear anomalies are detected in correspondence to the solid-state transformations. The Seebeck coefficient, measured as a function of temperature, highlights a conduction of n-type. The temperature dependence of the magnetic susceptibility displays an overall constant diamagnetic response.
Keywords: X-ray diffraction; copper arsenides; differential scanning calorimetry; electrical resistivity; first-order structural transition; lonsdaleite sublattice; magnetic susceptibility.
Conflict of interest statement
The authors do not have competing financial interest to declare.
Figures
















References
-
- Heyding R.D., Despault G.J.G. The copper/arsenic system and the copper arsenide minerals. Can. J. Chem. 1960;38:2477–2481. doi: 10.1139/v60-335. - DOI
-
- Mödlinger M., Cziegler A., Macció D., Schnideritsch H., Sabatini B. Archaeological Arsenical Bronzes and Equilibrium in the As-Cu System. Met. Mater. Trans. B. 2018;49:2505–2513. doi: 10.1007/s11663-018-1322-8. - DOI
-
- Subramanian P.R., Laughlin D.E. The As−Cu (Arsenic-Copper) system. Bull. Alloy Phase Diagr. 1988;9:605–618. doi: 10.1007/BF02881964. - DOI
-
- Naud J., Priest P. Contribution a l’etude du systeme cuivre-arsenic. Mater. Res. Bull. 1972;7:783–792. doi: 10.1016/0025-5408(72)90128-6. - DOI
-
- Brauer G., Zintl E. Konstitution von Phosphiden, Arseniden, Antimoniden und Wismutiden des Lithiums, Natriums und Kaliums. Z. Phys. Chem. Abstr. B. 1937;37:323–352. doi: 10.1515/zpch-1937-3725. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

