Recovery from Exercise in Persons with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
- PMID: 36984572
- PMCID: PMC10059925
- DOI: 10.3390/medicina59030571
Recovery from Exercise in Persons with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
Abstract
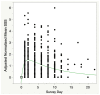
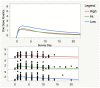
Background and Objectives: Post-exertional malaise (PEM) is the hallmark of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), but there has been little effort to quantitate the duration of PEM symptoms following a known exertional stressor. Using a Symptom Severity Scale (SSS) that includes nine common symptoms of ME/CFS, we sought to characterize the duration and severity of PEM symptoms following two cardiopulmonary exercise tests separated by 24 h (2-day CPET). Materials and Methods: Eighty persons with ME/CFS and 64 controls (CTL) underwent a 2-day CPET. ME/CFS subjects met the Canadian Clinical Criteria for diagnosis of ME/CFS; controls were healthy but not participating in regular physical activity. All subjects who met maximal effort criteria on both CPETs were included. SSS scores were obtained at baseline, immediately prior to both CPETs, the day after the second CPET, and every two days after the CPET-1 for 10 days. Results: There was a highly significant difference in judged recovery time (ME/CFS = 12.7 ± 1.2 d; CTL = 2.1 ± 0.2 d, mean ± s.e.m., Chi2 = 90.1, p < 0.0001). The range of ME/CFS patient recovery was 1-64 days, while the range in CTL was 1-10 days; one subject with ME/CFS had not recovered after one year and was not included in the analysis. Less than 10% of subjects with ME/CFS took more than three weeks to recover. There was no difference in recovery time based on the level of pre-test symptoms prior to CPET-1 (F = 1.12, p = 0.33). Mean SSS scores at baseline were significantly higher than at pre-CPET-1 (5.70 ± 0.16 vs. 4.02 ± 0.18, p < 0.0001). Pharmacokinetic models showed an extremely prolonged decay of the PEM response (Chi2 > 22, p < 0.0001) to the 2-day CPET. Conclusions: ME/CFS subjects took an average of about two weeks to recover from a 2-day CPET, whereas sedentary controls needed only two days. These data quantitate the prolonged recovery time in ME/CFS and improve the ability to obtain well-informed consent prior to doing exercise testing in persons with ME/CFS. Quantitative monitoring of PEM symptoms may provide a method to help manage PEM.
Keywords: 2-day cardiopulmonary exercise test; chronic fatigue syndrome; exercise recovery; myalgic encephalomyelitis; post-exertional malaise; specific symptom severity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Carruthers B.M., Jain A.K., De Meirleir K.L., Peterson D.L., Klimas N.G., Lerner A.M., Bested A.C., Flor-Henry P., Joshi P., Powles A.P., et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case definition, diagnostic and treatment protocols. J. Chronic Fatigue Syndr. 2003;11:7–115. doi: 10.1300/J092v11n01_02. - DOI
-
- IOM (Institute of Medicine) Beyond Myalgic Encepha-Lomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. The National Academies Press; Washington, DC, USA: 2015. - PubMed
-
- Lien K., Johansen B., Veierød M.B., Haslestad A.S., Bøhn S.K., Melsom M.N., Kardel K.R., Iversen P.O. Abnormal blood lactate accumulation during repeated exercise testing in myalgic encephalomyelitis/chronic fatigue syndrome. Physiol. Rep. 2019;7:14138. doi: 10.14814/phy2.14138. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

