Improving Structural Homogeneity, Hydraulic Permeability, and Mechanical Performance of Asymmetric Monophasic Cellulose Acetate/Silica Membranes: Spinodal Decomposition Mix
- PMID: 36984734
- PMCID: PMC10059883
- DOI: 10.3390/membranes13030346
Improving Structural Homogeneity, Hydraulic Permeability, and Mechanical Performance of Asymmetric Monophasic Cellulose Acetate/Silica Membranes: Spinodal Decomposition Mix
Abstract
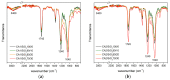
In this paper, we propose an optimized protocol to synthesize reproducible, accurate, sustainable integrally skinned monophasic hybrid cellulose acetate/silica membranes for ultrafiltration. Eight different membrane compositions were studied, divided into two series, one and two, each composed of four membranes. The amount of silica increased from 0 wt.% up to 30 wt.% (with increments of 10 wt.%) in each series, while the solvent composition was kept constant within each series (formamide/acetone ratio equals 0.57 wt.% in series one and 0.73 wt.% in series two). The morphology of the membranes was analyzed by scanning electron microscopy and the chemical composition by Fourier transform infrared spectroscopy, in attenuated total reflection mode (FTIR-ATR). Mechanical tensile properties were determined using tensile tests, and a retest trial was performed to assess mechanical properties variability over different membrane batches. The hydraulic permeability of the membranes was evaluated by measuring pure water fluxes following membrane compaction. The membranes in series two produced with a higher formamide/acetone solvent ratio led to thicker membranes with higher hydraulic permeability values (47.2-26.39 kg·h-1·m-2·bar-1) than for the membranes in series one (40.01-19.4 kg·h-1·m-2·bar-1). Results obtained from the FTIR-ATR spectra suggest the presence of micro/nano-silica clusters in the hybrid membranes of series one, also exhibiting higher Young's modulus values than the hybrid membranes in series two.
Keywords: CA/SiO2 membrane; hydraulic permeability; integral asymmetric membranes; mechanical tensile properties; monophasic hybrid membrane; spinodal decompositions mix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












Similar articles
-
Structure of water in hybrid cellulose acetate-silica ultrafiltration membranes and permeation properties.Carbohydr Polym. 2018 Jun 1;189:342-351. doi: 10.1016/j.carbpol.2018.02.030. Epub 2018 Feb 16. Carbohydr Polym. 2018. PMID: 29580418
-
Synthesis and Characterization of Novel Integral Asymmetric Monophasic Cellulose-Acetate/Silica/Titania and Cellulose-Acetate/Titania Membranes.Membranes (Basel). 2020 Aug 20;10(9):195. doi: 10.3390/membranes10090195. Membranes (Basel). 2020. PMID: 32825422 Free PMC article.
-
Novel Cellulose Acetate-Based Monophasic Hybrid Membranes for Improved Blood Purification Devices: Characterization under Dynamic Conditions.Membranes (Basel). 2021 Oct 27;11(11):825. doi: 10.3390/membranes11110825. Membranes (Basel). 2021. PMID: 34832054 Free PMC article.
-
Improving hydraulic permeability, mechanical properties, and chemical functionality of cellulose acetate-based membranes by co-polymerization with tetraethyl orthosilicate and 3-(aminopropyl)triethoxysilane.Carbohydr Polym. 2021 Jun 1;261:117813. doi: 10.1016/j.carbpol.2021.117813. Epub 2021 Feb 20. Carbohydr Polym. 2021. PMID: 33766330
-
Enhanced permeability and antifouling performance of cellulose acetate ultrafiltration membrane assisted by l-DOPA functionalized halloysite nanotubes.Carbohydr Polym. 2017 Oct 15;174:688-696. doi: 10.1016/j.carbpol.2017.06.089. Epub 2017 Jun 24. Carbohydr Polym. 2017. PMID: 28821120 Review.
Cited by
-
Low-Alpha-Cellulose-Based Membranes.Polymers (Basel). 2025 Feb 24;17(5):598. doi: 10.3390/polym17050598. Polymers (Basel). 2025. PMID: 40076091 Free PMC article.
References
-
- Pendergast M.M., Hoek E.M.V. A Review of Water Treatment Membrane Nanotechnologies. Energy Environ. Sci. 2011;4:1946–1971. doi: 10.1039/c0ee00541j. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

