Hydrogen Sulphide Sequestration with Metallic Ions in Acidic Media Based on Chitosan/sEPDM/Polypropylene Composites Hollow Fiber Membranes System
- PMID: 36984736
- PMCID: PMC10057485
- DOI: 10.3390/membranes13030350
Hydrogen Sulphide Sequestration with Metallic Ions in Acidic Media Based on Chitosan/sEPDM/Polypropylene Composites Hollow Fiber Membranes System
Abstract
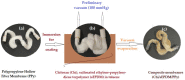
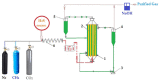
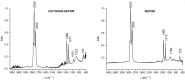
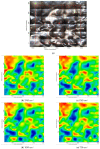
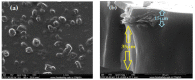
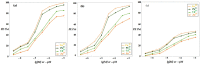
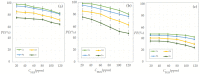
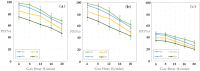
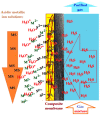
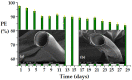
This paper presents the preparation and characterization of composite membranes based on chitosan (Chi), sulfonated ethylene-propylene-diene terpolymer (sEPDM), and polypropylene (PPy), and designed to capture hydrogen sulfide. The Chi/sEPDM/PPy composite membranes were prepared through controlled evaporation of a toluene dispersion layer of Chi:sEPDM 1;1, w/w, deposited by immersion and under a slight vacuum (100 mmHg) on a PPy hollow fiber support. The composite membranes were characterized morphologically, structurally, and thermally, but also from the point of view of their performance in the process of hydrogen sulfide sequestration in an acidic media solution with metallic ion content (Cu2+, Cd2+, Pb2+, and/or Zn2+). The operational parameters of the pertraction were the pH, pM, matrix gas flow rate, and composition. The results of pertraction from synthetic gases mixture (nitrogen, methane, carbon dioxide) indicated an efficient removal of hydrogen sulfide through the prepared composite membranes, as well as its immobilization as sulfides. The sequestration and the recuperative separation, as sulfides from an acid medium, of the hydrogen sulfide reached up to 96%, decreasing in the order: CuS > PbS > CdS > ZnS.
Keywords: chitosan; composite membranes; electronics waste; hydrogen sulphide sequestration; polypropylene hollow fiber membrane; sEPDM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Nechifor A.C., Cotorcea S., Bungău C., Albu P.C., Pașcu D., Oprea O., Grosu A.R., Pîrțac A., Nechifor G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes. 2021;11:256. doi: 10.3390/membranes11040256. - DOI - PMC - PubMed
-
- Li H., Lu J., Li B. Does pollution-intensive industrial agglomeration increase residents’ health expenditure? Sustain. Cities Soc. 2020;56:102092. doi: 10.1016/j.scs.2020.102092. - DOI
-
- Pan Y., Guo J., Yang L., Yuan Q., Ren Z., Wang L. Numerical Simulations of Non-Point Source Pollution in a Small Urban Catchment: Identification of Pollution Risk Areas and Effectiveness of Source-Control Measures. Water. 2021;13:96. doi: 10.3390/w13010096. - DOI
-
- Yu S., Bao J., Ding W., Chen X., Tang X., Hao J., Zhang W., Singh P. Investigating the Relationship between Public Satisfaction and Public Environmental Participation during Government Treatment of Urban Malodorous Black River in China. Sustainability. 2021;13:3584. doi: 10.3390/su13063584. - DOI
LinkOut - more resources
Full Text Sources

