The Impact of Nutrient Intake and Metabolic Wastes during Pregnancy on Offspring Hypertension: Challenges and Future Opportunities
- PMID: 36984857
- PMCID: PMC10052993
- DOI: 10.3390/metabo13030418
The Impact of Nutrient Intake and Metabolic Wastes during Pregnancy on Offspring Hypertension: Challenges and Future Opportunities
Abstract
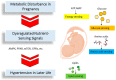
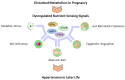
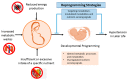
Hypertension can have its origin in early life. During pregnancy, many metabolic alterations occur in the mother that have a crucial role in fetal development. In response to maternal insults, fetal programming may occur after metabolic disturbance, resulting in programmed hypertension later in life. Maternal dietary nutrients act as metabolic substrates for various metabolic processes via nutrient-sensing signals. Different nutrient-sensing pathways that detect levels of sugars, amino acids, lipids and energy are integrated during pregnancy, while disturbed nutrient-sensing signals have a role in the developmental programming of hypertension. Metabolism-modulated metabolites and nutrient-sensing signals are promising targets for new drug discovery due to their pathogenic link to hypertension programming. Hence, in this review, we pay particular attention to the maternal nutritional insults and metabolic wastes affecting fetal programming. We then discuss the role of nutrient-sensing signals linking the disturbed metabolism to hypertension programming. This review also summarizes current evidence to give directions for future studies regarding how to prevent hypertension via reprogramming strategies, such as nutritional intervention, targeting nutrient-sensing signals, and reduction of metabolic wastes. Better prevention for hypertension may be possible with the help of novel early-life interventions that target altered metabolism.
Keywords: AMP-activated protein kinase (AMPK); asymmetric dimethylarginine; developmental origins of health and disease (DOHaD); hypertension; nutrient-sensing signal; short chain fatty acid; trimethylamine-N-oxide; uremic toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Interplay between Oxidative Stress and Nutrient Sensing Signaling in the Developmental Origins of Cardiovascular Disease.Int J Mol Sci. 2017 Apr 15;18(4):841. doi: 10.3390/ijms18040841. Int J Mol Sci. 2017. PMID: 28420139 Free PMC article. Review.
-
The Interplay between Maternal and Post-Weaning High-Fat Diet and Gut Microbiota in the Developmental Programming of Hypertension.Nutrients. 2019 Aug 22;11(9):1982. doi: 10.3390/nu11091982. Nutrients. 2019. PMID: 31443482 Free PMC article.
-
Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: interplay between nutrient-sensing signals, oxidative stress and gut microbiota.J Nutr Biochem. 2019 Aug;70:28-37. doi: 10.1016/j.jnutbio.2019.04.002. Epub 2019 Apr 24. J Nutr Biochem. 2019. PMID: 31108332
-
PPARs Link Early Life Nutritional Insults to Later Programmed Hypertension and Metabolic Syndrome.Int J Mol Sci. 2015 Dec 24;17(1):20. doi: 10.3390/ijms17010020. Int J Mol Sci. 2015. PMID: 26712739 Free PMC article. Review.
-
Developmental Programming and Reprogramming of Hypertension and Kidney Disease: Impact of Tryptophan Metabolism.Int J Mol Sci. 2020 Nov 18;21(22):8705. doi: 10.3390/ijms21228705. Int J Mol Sci. 2020. PMID: 33218054 Free PMC article. Review.
Cited by
-
Maternal High-Fat Diet Controls Offspring Kidney Health and Disease.Nutrients. 2023 Jun 9;15(12):2698. doi: 10.3390/nu15122698. Nutrients. 2023. PMID: 37375602 Free PMC article. Review.
-
Pregnancy Metabolic Adaptation and Changes in Placental Metabolism in Preeclampsia.Geburtshilfe Frauenheilkd. 2024 Sep 19;84(11):1033-1042. doi: 10.1055/a-2403-4855. eCollection 2024 Nov. Geburtshilfe Frauenheilkd. 2024. PMID: 39524034 Free PMC article.
References
-
- GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–1994. - PMC - PubMed
-
- Baker-Smith C.M., Flinn S.K., Flynn J.T., Kaelber D.C., Blowey D., Carroll A.E., Daniels S.R., de Ferranti S.D., Dionne J.M., Falkner B., et al. Diagnosis, Evaluation, and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2018;142:e20182096. doi: 10.1542/peds.2018-2096. - DOI - PubMed
-
- Mills K.T., Bundy J.D., Kelly T.N., Reed J.E., Kearney P.M., Reynolds K., Chen J., He J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

