Overexpression of Bacterial Beta-Ketothiolase Improves Flax (Linum usitatissimum L.) Retting and Changes the Fibre Properties
- PMID: 36984877
- PMCID: PMC10052753
- DOI: 10.3390/metabo13030437
Overexpression of Bacterial Beta-Ketothiolase Improves Flax (Linum usitatissimum L.) Retting and Changes the Fibre Properties
Abstract
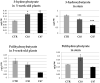
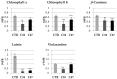
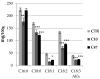
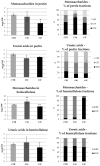
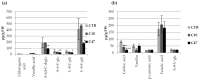
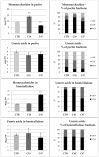
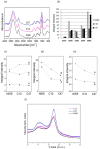
Beta-ketothiolases are involved in the beta-oxidation of fatty acids and the metabolism of hormones, benzenoids, and hydroxybutyrate. The expression of bacterial beta-ketothiolase in flax (Linum usitatissimum L.) results in an increase in endogenous beta-ketothiolase mRNA levels and beta-hydroxybutyrate content. In the present work, the effect of overexpression of beta-ketothiolase on retting and stem and fibre composition of flax plants is presented. The content of the components was evaluated by high-performance liquid chromatography, gas chromatography-mass spectrometry, Fourier-transform infrared spectroscopy, and biochemical methods. Changes in the stem cell walls, especially in the lower lignin and pectin content, resulted in more efficient retting. The overexpression of beta-ketothiolase reduced the fatty acid and carotenoid contents in flax and affected the distribution of phenolic compounds between free and cell wall-bound components. The obtained fibres were characterized by a slightly lower content of phenolic compounds and changes in the composition of the cell wall. Based on the IR analysis, we concluded that the production of hydroxybutyrate reduced the cellulose crystallinity and led to the formation of shorter but more flexible cellulose chains, while not changing the content of the cell wall components. We speculate that the changes in chemical composition of the stems and fibres are the result of the regulatory properties of hydroxybutyrate. This provides us with a novel way to influence metabolic composition in agriculturally important crops.
Keywords: 3-hydroxybutyrate; beta-ketothiolase; cell wall; flax fibre; flax stem; polyhydroxybutyrate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
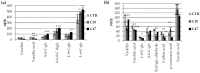
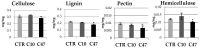

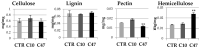
Similar articles
-
Improving retting of fibre through genetic modification of flax to express pectinases.Transgenic Res. 2008 Feb;17(1):133-47. doi: 10.1007/s11248-007-9080-4. Epub 2007 Mar 20. Transgenic Res. 2008. PMID: 17372706
-
Lignin deficiency in transgenic flax resulted in plants with improved mechanical properties.J Biotechnol. 2007 Mar 10;128(4):919-34. doi: 10.1016/j.jbiotec.2006.12.030. Epub 2007 Jan 17. J Biotechnol. 2007. PMID: 17280732
-
Fibres from flax overproducing β-1,3-glucanase show increased accumulation of pectin and phenolics and thus higher antioxidant capacity.BMC Biotechnol. 2013 Feb 9;13:10. doi: 10.1186/1472-6750-13-10. BMC Biotechnol. 2013. PMID: 23394294 Free PMC article.
-
Linen most useful: perspectives on structure, chemistry, and enzymes for retting flax.ISRN Biotechnol. 2012 Dec 30;2013:186534. doi: 10.5402/2013/186534. eCollection 2013. ISRN Biotechnol. 2012. PMID: 25969769 Free PMC article. Review.
-
Secondary cell-wall assembly in flax phloem fibres: role of galactans.Planta. 2006 Jan;223(2):149-58. doi: 10.1007/s00425-005-0118-7. Epub 2005 Dec 16. Planta. 2006. PMID: 16362330 Review.
References
-
- Preisner M., Wojtasik W., Kulma A., Żuk M., Szopa J. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons; Hoboken, NJ, USA: 2014. Flax Fiber.
-
- Kulma A., Skórkowska-Telichowska K., Kostyn K., Szatkowski M., Skała J., Drulis-Kawa Z., Preisner M., Żuk M., Szperlik J., Wang Y.F., et al. New Flax Producing Bioplastic Fibers for Medical Purposes. Ind. Crops Prod. 2015;68:80–89. doi: 10.1016/j.indcrop.2014.09.013. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

