3D-Printed Microfluidic Chip for Real-Time Glucose Monitoring in Liquid Analytes
- PMID: 36984909
- PMCID: PMC10052769
- DOI: 10.3390/mi14030503
3D-Printed Microfluidic Chip for Real-Time Glucose Monitoring in Liquid Analytes
Abstract
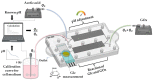
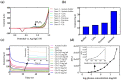
The connection of macrosystems with microsystems for in-line measurements is important in different biotechnological processes as it enables precise and accurate monitoring of process parameters at a small scale, which can provide valuable insights into the process, and ultimately lead to improved process control and optimization. Additionally, it allows continuous monitoring without the need for manual sampling and analysis, leading to more efficient and cost-effective production. In this paper, a 3D printed microfluidic (MF) chip for glucose (Glc) sensing in a liquid analyte is proposed. The chip made in Poly(methyl methacrylate) (PMMA) contains integrated serpentine-based micromixers realized via stereolithography with a slot for USB-like integration of commercial DropSens electrodes. After adjusting the sample's pH in the first micromixer, small volumes of the sample and enzyme are mixed in the second micromixer and lead to a sensing chamber where the Glc concentration is measured via chronoamperometry. The sensing potential was examined for Glc concentrations in acetate buffer in the range of 0.1-100 mg/mL and afterward tested for Glc sensing in a cell culturing medium. The proposed chip showed great potential for connection with macrosystems, such as bioreactors, for direct in-line monitoring of a quality parameter in a liquid sample.
Keywords: 3D printing; PMMA; SLA; electrochemical sensor; glucose; lab-on-a-chip; microfluidics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Convery N., Gadegaard N. 30 years of microfluidics. Micro Nano Eng. 2019;2:76–91. doi: 10.1016/j.mne.2019.01.003. - DOI
-
- Preetam S., Nahak B.K., Patra S., Toncu D.C., Park S., Syväjärvi M., Orive G., Tiwari A. Emergence of microfluidics for next generation biomedical devices. Biosens. Bioelectron. X. 2022;10:100106. doi: 10.1016/j.biosx.2022.100106. - DOI
-
- Akbari Kenari M., Rezvani Ghomi E., Akbari Kenari A., Arabi S.M.S., Deylami J., Ramakrishna S. Biomedical applications of microfluidic devices: Achievements and challenges. Polym. Adv. Technol. 2022;33:3920–3934. doi: 10.1002/pat.5847. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

