The Pleiotropic Effects of Carbohydrate-Mediated Growth Rate Modifications in Bifidobacterium longum NCC 2705
- PMID: 36985162
- PMCID: PMC10059941
- DOI: 10.3390/microorganisms11030588
The Pleiotropic Effects of Carbohydrate-Mediated Growth Rate Modifications in Bifidobacterium longum NCC 2705
Abstract
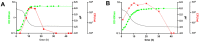
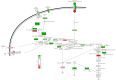
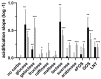
Bifidobacteria are saccharolytic bacteria that are able to metabolize a relatively large range of carbohydrates through their unique central carbon metabolism known as the "bifid-shunt". Carbohydrates have been shown to modulate the growth rate of bifidobacteria, but unlike for other genera (e.g., E. coli or L. lactis), the impact it may have on the overall physiology of the bacteria has not been studied in detail to date. Using glucose and galactose as model substrates in Bifidobacterium longum NCC 2705, we established that the strain displayed fast and slow growth rates on those carbohydrates, respectively. We show that these differential growth conditions are accompanied by global transcriptional changes and adjustments of central carbon fluxes. In addition, when grown on galactose, NCC 2705 cells were significantly smaller, exhibited an expanded capacity to import and metabolized different sugars and displayed an increased acid-stress resistance, a phenotypic signature associated with generalized fitness. We predict that part of the observed adaptation is regulated by the previously described bifidobacterial global transcriptional regulator AraQ, which we propose to reflect a catabolite-repression-like response in B. longum. With this manuscript, we demonstrate that not only growth rate but also various physiological characteristics of B. longum NCC 2705 are responsive to the carbon source used for growth, which is relevant in the context of its lifestyle in the human infant gut where galactose-containing oligosaccharides are prominent.
Keywords: bifidobacteria; carbon metabolism; catabolite repression; growth rate; stress resistance.
Conflict of interest statement
S. Duboux, S. Pruvost, C. Joyce, B. Bogicevic and J.A. Muller are all employed by Société des produits Nestlé SA.
Figures



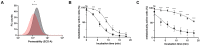
References
-
- Fujimura K.E., Sitarik A.R., Havstad S., Lin D.L., LeVan S., Fadrosh D., Panzer A.R., LaMere B., Rackaityte E., Lukacs N.W., et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016;22:1187–1191. doi: 10.1038/nm.4176. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

