Effects of Cysticercus cellulosae Excretory-Secretory Antigens on the TGF-β Signaling Pathway and Th17 Cell Differentiation in Piglets, a Proteomic Analysis
- PMID: 36985175
- PMCID: PMC10056668
- DOI: 10.3390/microorganisms11030601
Effects of Cysticercus cellulosae Excretory-Secretory Antigens on the TGF-β Signaling Pathway and Th17 Cell Differentiation in Piglets, a Proteomic Analysis
Abstract
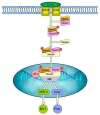
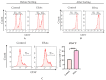
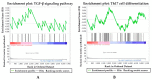
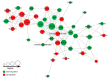
Excretory-secretory antigens (ESAs) of Cysticercus cellulosae can directly regulate the proliferation and differentiation of host T regulatory (Treg) cells, thus inhibiting host immune responses. However, previous studies have only focused on this phenomenon, and the molecular mechanisms behind the ways in which C. cellulosae ESAs regulate the differentiation of host Treg/Th17 cells have not been reported. We collected CD3+ T cells stimulated by C. cellulosae ESAs through magnetic bead sorting and used label-free quantification (LFQ) proteomics techniques to analyze the signaling pathways of C. cellulosae ESAs regulating Treg/Th17 cell differentiation. Through gene set enrichment analysis (GSEA), we found that C. cellulosae ESAs could upregulate the TGF-β signaling pathway and downregulate Th17 cell differentiation in piglet T cells. Interestingly, we also found that the IL-2/STAT5 signaling pathway also affects the downregulation of Th17 cell differentiation. C. cellulosae ESAs activate the TGF-β signaling pathway and the IL-2/STAT5 signaling pathway in host T cells to further regulate the differentiation of Treg/Th17 cells in order to evade host immune attack. This study lays the foundation for the subsequent verification of these pathways, and further clarifies the molecular mechanism of C. cellulosae-mediated immune evasion.
Keywords: TGF-β signaling pathway; Th17 cells differentiation; excretory–secretory antigens; proteomics.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Himwaze C., Mucheleng’anga L.A., Telendiy V., Hamukale A., Tembo J., Kapata N., Ntoumi F., Zumla A. Cardiac cysticercosis and neurocysticercosis in sudden and unexpected community deaths in Lusaka, Zambia: A descriptive medico-legal post-mortem examination study. Int. J. Infect. Dis. 2022;115:195–200. doi: 10.1016/j.ijid.2021.11.042. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

