Nanoparticle Coatings on Glass Surfaces to Prevent Pseudomonas fluorescens AR 11 Biofilm Formation
- PMID: 36985196
- PMCID: PMC10057769
- DOI: 10.3390/microorganisms11030621
Nanoparticle Coatings on Glass Surfaces to Prevent Pseudomonas fluorescens AR 11 Biofilm Formation
Abstract
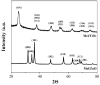
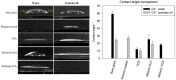
Microbial colonization of surfaces is a sanitary and industrial issue for many applications, leading to product contamination and human infections. When microorganisms closely interact with a surface, they start to produce an exo-polysaccaridic matrix to adhere to and protect themselves from adverse environmental conditions. This type of structure is called a biofilm. The aim of our work is to investigate novel technologies able to prevent biofilm formation by surface coatings. We coated glass surfaces with melanin-ZnO2, melanin-TiO2, and TiO2 hybrid nanoparticles. The functionalization was performed using cold plasma to activate glass-substrate-coated surfaces, that were characterized by performing water and soybean oil wetting tests. A quantitative characterization of the antibiofilm properties was done using Pseudomonas fluorescens AR 11 as a model organism. Biofilm morphologies were observed using confocal laser scanning microscopy and image analysis techniques were used to obtain quantitative morphological parameters. The results highlight the efficacy of the proposed surface coating to prevent biofilm formation. Melanin-TiO2 proved to be the most efficient among the particles investigated. Our results can be a valuable support for future implementation of the technique proposed here in an extended range of applications that may include further testing on other strains and other support materials.
Keywords: antibiofilm; antimicrobial; biofilm; cold plasma; confocal laser scanning microscopy; hybrid nanoparticles; wetting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zabiegaj D., Hajirasouliha F., Duilio A., Guido S., Caserta S., Kostoglou M., Petala M., Karapantsios T., Trybala A. Wetting/spreading on porous media and on deformable, soluble structured substrates as a model system for studying the effect of morphology on biofilms wetting and for assessing anti-biofilm methods. Curr. Opin. Colloid Interface Sci. 2021;53:101426. doi: 10.1016/j.cocis.2021.101426. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

