Methylprednisolone Promotes Mycobacterium smegmatis Survival in Macrophages through NF-κB/DUSP1 Pathway
- PMID: 36985341
- PMCID: PMC10058212
- DOI: 10.3390/microorganisms11030768
Methylprednisolone Promotes Mycobacterium smegmatis Survival in Macrophages through NF-κB/DUSP1 Pathway
Abstract
Background: Mycobacterium tuberculosis (M. tuberculosis) is the causative agent of tuberculosis. As an important component of host immunity, macrophages are not only the first line of defense against M. tuberculosis but also the parasitic site of M. tuberculosis in the host. Glucocorticoids can cause immunosuppression, which is considered to be one of the major risk factors for active tuberculosis, but the mechanism is unclear.
Objective: To study the effect of methylprednisolone on the proliferation of mycobacteria in macrophages and try to find key molecules of this phenomenon.
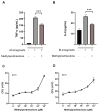
Methods: The macrophage line RAW264.7 infected by M. smegmatis was treated with methylprednisolone, and the intracellular bacterial CFU, Reactive Oxygen Species (ROS), cytokine secretion, autophagy, and apoptosis were measured. After the cells were treated with NF-κB inhibitor BAY 11-7082 and DUSP1 inhibitor BCI, respectively, the intracellular bacterial CFU, ROS, IL-6, and TNF-α secretion were detected.
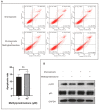
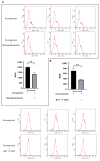
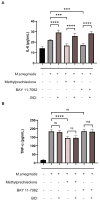
Results: After treatment with methylprednisolone, the CFU of intracellular bacteria increased, the level of ROS decreased, and the secretion of IL-6 and TNF-α decreased in infected macrophages. After BAY 11-7082 treatment, the CFU of M. smegmatis in macrophages increased, and the level of ROS production and the secretion of IL-6 by macrophages decreased. Transcriptome high-throughput sequencing and bioinformatics analysis suggested that DUSP1 was the key molecule in the above phenomenon. Western blot analysis confirmed that the expression level of DUSP1 was increased in the infected macrophages treated with methylprednisolone and BAY 11-7082, respectively. After BCI treatment, the level of ROS produced by infected macrophages increased, and the secretion of IL-6 increased. After the treatment of BCI combined with methylprednisolone or BAY 11-7082, the level of ROS produced and the secretion of IL-6 by macrophages were increased.
Conclusion: methylprednisolone promotes the proliferation of mycobacteria in macrophages by suppressing cellular ROS production and IL-6 secretion through down-regulating NF-κB and up-regulating DUSP1 expression. BCI, an inhibitor of DUSP1, can reduce the level of DUSP1 in the infected macrophages and inhibit the proliferation of intracellular mycobacteria by promoting cellular ROS production and IL-6 secretion. Therefore, BCI may become a new molecule for host-directed therapy of tuberculosis, as well as a new strategy for the prevention of tuberculosis when treated with glucocorticoids.
Keywords: DUSP1; IL-6; NF-κB; ROS; methylprednisolone; mycobacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- World Health Organization Global Tuberculosis Report 2021. 2021. [(accessed on 1 December 2022)]. Available online: https://www.who.int/publications/i/item/9789240037021.
-
- World Health Organization Global Tuberculosis Report 2015. 2015. [(accessed on 1 December 2022)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/191102/9789241565059_en....
-
- Getahun H., Matteelli A., Abubakar I., Aziz M., Baddeley A., Barreira D., Den Boon S., Borroto Gutierrez S., Bruchfeld J., Burhan E., et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur. Respir. J. 2015;46:1563–1576. doi: 10.1183/13993003.01245-2015. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

