Challenges and Recent Advancements in COVID-19 Vaccines
- PMID: 36985360
- PMCID: PMC10059828
- DOI: 10.3390/microorganisms11030787
Challenges and Recent Advancements in COVID-19 Vaccines
Abstract
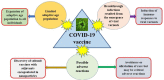
Vaccination is the most effective method for the prevention of COVID-19 caused by SARS-CoV-2, which is still a global epidemic. However, the evolution of SARS-CoV-2 is so rapid that various variants, including the Alpha, Beta, Gamma, Delta, and Omicron variants, have emerged, lowering the protection rate of vaccines and even resulting in breakthrough infections. Additionally, some rare but severe adverse reactions induced by COVID-19 vaccines may raise safety concerns and hinder vaccine promotion; however, clinical studies have shown that the benefits of vaccination outweigh the risks caused by adverse reactions. Current vaccines approved with emergency use authorization (EUA) were originally adaptive for adults only, and infants, children, and adolescents are not included. New-generation vaccines are needed to overcome the challenges of limited adaptive age population, breakthrough infection (mainly due to virus variant emergencies), and critical adverse reactions. Fortunately, some advances in COVID-19 vaccines have been obtained regarding enlarged adaptive populations for clinical applications, such as the Pfizer/BioNTech vaccine and the Moderna vaccine. In this article, we provide a review on the challenges and recent advancements in COVID-19 vaccines. The development of next-generation COVID-19 vaccines should lay emphasis on the expansion of adaptive age populations in all individuals, the induction of immune responses to viral variants, the avoidance or alleviation of rare but potentially critical adverse reactions, and the discovery of subunit vaccines with adjuvants encapsulated in nanoparticles.
Keywords: COVID-19 vaccine; EUA; SARS-CoV-2; adverse reaction; breakthrough infection; viral variant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Malik Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020;42:3–11. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous