Synthesis of Phosphorus(V)-Substituted Six-Membered N-Heterocycles: Recent Progress and Challenges
- PMID: 36985443
- PMCID: PMC10054050
- DOI: 10.3390/molecules28062472
Synthesis of Phosphorus(V)-Substituted Six-Membered N-Heterocycles: Recent Progress and Challenges
Abstract
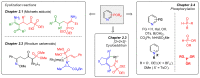
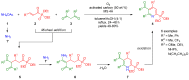
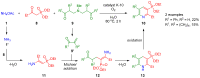
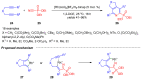
Heterocycles functionalized with pentavalent phosphorus are of great importance since they include a great variety of biologically active compounds and pharmaceuticals, advanced materials, and valuable reactive intermediates for organic synthesis. Significant progress in synthesis of P(O)R2-substituted six-membered heterocycles has been made in the past decade. This review covers the synthetic strategies towards aromatic monocyclic six-membered N-heterocycles, such as pyridines, pyridazines, pyrimidines, and pyrazines bearing phosphonates and phosphine oxides, which were reported from 2012 to 2022.
Keywords: heterocycles; organophosphorus compounds; pyrazine; pyridazine; pyridine; pyrimidine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



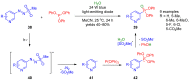
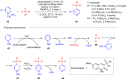
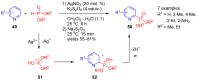

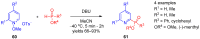
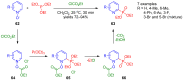

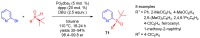


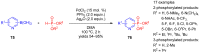

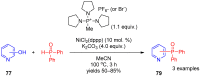




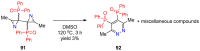

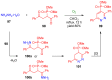
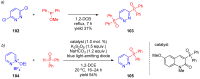
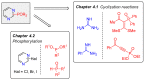




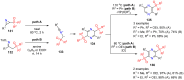


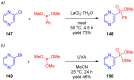
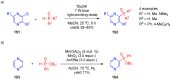

References
-
- Nixon T.D., Gamble A.J., Thatcher R.J., Whitwood A.C., Lynam J.M. Synthesis and coordination chemistry of pyrimidine-substituted phosphine ligands. Inorg. Chim. Acta. 2012;380:252–260. doi: 10.1016/j.ica.2011.10.058. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

