Supermolecule-Drug Conjugates Based on Acid-Degradable Polyrotaxanes for pH-Dependent Intracellular Release of Doxorubicin
- PMID: 36985487
- PMCID: PMC10056152
- DOI: 10.3390/molecules28062517
Supermolecule-Drug Conjugates Based on Acid-Degradable Polyrotaxanes for pH-Dependent Intracellular Release of Doxorubicin
Abstract
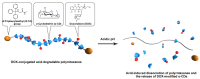
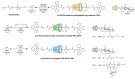
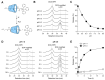
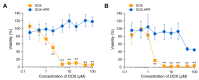
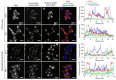
Doxorubicin (DOX)-conjugated acid-degradable polyrotaxanes (PRXs) were designed as supramolecular drug carriers capable of releasing drugs in acidic cellular environments. Acid-degradable PRXs composed of α-cyclodextrin (α-CD) as a cyclic molecule, poly(ethylene glycol) (PEG) as a polymer axis, and N-triphenylmethyl (N-Trt) groups as an acid-labile stopper molecules were synthesized and DOX was conjugated with the threaded α-CDs in the PRXs. Because the acid-induced cleavage of N-Trt groups in PRXs leads to PRX dissociation, the DOX-modified α-CDs were released under acidic conditions (pH 5.0). The cytotoxicity of DOX-conjugated PRXs in colon-26 cells revealed significant cell death for DOX-conjugated PRXs after 48 h of treatment. Confocal laser scanning microscopy (CLSM) analysis revealed that the fluorescence signals derived from DOX-conjugated PRXs were observed in cellular nuclei after 48 h, suggesting that the DOX-modified α-CDs were released and accumulated in cellular nuclei. These results confirmed that acid-degradable PRXs can be utilized as drug carriers capable of releasing drug-modified α-CDs in acidic lysosomes and eliciting cytotoxicity. Overall, acid-degradable PRXs represent a promising supramolecular framework for the delivery and intracellular release of drug-modified α-CDs, and PRX-drug conjugates are expected to contribute to the development of pH-responsive drug carriers for cancer therapy.
Keywords: cyclodextrin; doxorubicin; drug delivery system; polyrotaxane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

