Catalytic Asymmetric α-Functionalization of α-Branched Aldehydes
- PMID: 36985666
- PMCID: PMC10056299
- DOI: 10.3390/molecules28062694
Catalytic Asymmetric α-Functionalization of α-Branched Aldehydes
Abstract
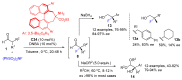
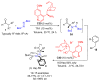
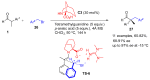
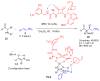
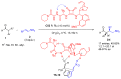
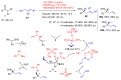
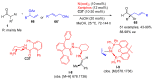
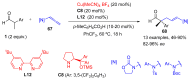
Aldehydes constitute a main class of organic compounds widely applied in synthesis. As such, catalyst-controlled enantioselective α-functionalization of aldehydes has attracted great interest over the years. In this context, α-branched aldehydes are especially challenging substrates because of reactivity and selectivity issues. Firstly, the transient trisubstituted enamines and enolates resulting upon treatment with an aminocatalyst or a base, respectively, would exhibit attenuated reactivity; secondly, mixtures of E- and Z-configured enamines/enolates may be formed; and third, effective face-discrimination on such trisubstituted sp2 carbon intermediates by the incoming electrophilic reagent is not trivial. Despite these issues, in the last 15 years, several catalytic approaches for the α-functionalization of prostereogenic α-branched aldehydes that proceed in useful yields and diastereo- and enantioselectivity have been uncovered. Developments include both organocatalytic and metal-catalyzed approaches as well as dual catalysis strategies for forging new carbon-carbon and carbon-heteroatom (C-O, N, S, F, Cl, Br, …) bond formation at Cα of the starting aldehyde. In this review, some key early contributions to the field are presented, but focus is on the most recent methods, mainly covering the literature from year 2014 onward.
Keywords: aldehydes; asymmetric catalysis; dual catalysis; organocatalysis; quaternary carbon; reaction umpolung.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







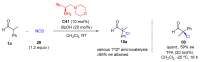






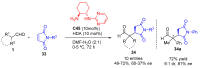




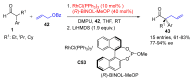








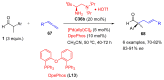















References
-
- Bella M., Gasperi T. Organocatalytic Formation of Quaternary Stereocenters. Synthesis. 2009;2009:1583–1614. doi: 10.1055/s-0029-1216796. - DOI
-
- Zhou F., Liu Y.-L., Zhou J. Catalytic Asymmetric Synthesis of Oxindoles Bearing a Tetrasubstituted Stereocenter at the C-3 Position. Adv. Synth. Catal. 2010;352:1381–1407. doi: 10.1002/adsc.201000161. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

