Mechanical Stimulation Decreases Auxin and Gibberellic Acid Synthesis but Does Not Affect Auxin Transport in Axillary Buds; It Also Stimulates Peroxidase Activity in Petunia × atkinsiana
- PMID: 36985685
- PMCID: PMC10053601
- DOI: 10.3390/molecules28062714
Mechanical Stimulation Decreases Auxin and Gibberellic Acid Synthesis but Does Not Affect Auxin Transport in Axillary Buds; It Also Stimulates Peroxidase Activity in Petunia × atkinsiana
Abstract
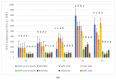
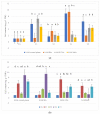
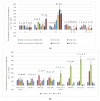
Thigmomorphogenesis (or mechanical stimulation-MS) is a term created by Jaffe and means plant response to natural stimuli such as the blow of the wind, strong rain, or touch, resulting in a decrease in length and an increase of branching as well as an increase in the activity of axillary buds. MS is very well known in plant morphology, but physiological processes controlling plant growth are not well discovered yet. In the current study, we tried to find an answer to the question if MS truly may affect auxin synthesis or transport in the early stage of plant growth, and which physiological factors may be responsible for growth arrest in petunia. According to the results of current research, we noticed that MS affects plant growth but does not block auxin transport from the apical bud. MS arrests IAA and GA3 synthesis in MS-treated plants over the longer term. The main factor responsible for the thickening of cell walls and the same strengthening of vascular tissues and growth arrestment, in this case, is peroxidase (POX) activity, but special attention should be also paid to AGPs as signaling molecules which also are directly involved in growth regulation as well as in cell wall modifications.
Keywords: cell wall lignification; plant architecture; plant hormone synthesis; thigmomorphogenesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Li Z.G., Gong M. Mechanical stimulation-induced cross-adaptation in plants: An overview. J. Plant Biol. 2011;54:358–364. doi: 10.1007/s12374-011-9178-3. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

