Ion-Imprinted Polymeric Materials for Selective Adsorption of Heavy Metal Ions from Aqueous Solution
- PMID: 36985770
- PMCID: PMC10055817
- DOI: 10.3390/molecules28062798
Ion-Imprinted Polymeric Materials for Selective Adsorption of Heavy Metal Ions from Aqueous Solution
Abstract
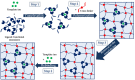
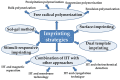
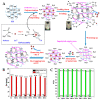
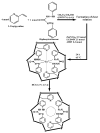
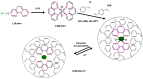
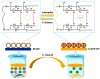
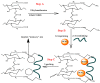
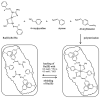
The introduction of selective recognition sites toward certain heavy metal ions (HMIs) is a great challenge, which has a major role when the separation of species with similar physicochemical features is considered. In this context, ion-imprinted polymers (IIPs) developed based on the principle of molecular imprinting methodology, have emerged as an innovative solution. Recent advances in IIPs have shown that they exhibit higher selectivity coefficients than non-imprinted ones, which could support a large range of environmental applications starting from extraction and monitoring of HMIs to their detection and quantification. This review will emphasize the application of IIPs for selective removal of transition metal ions (including HMIs, precious metal ions, radionuclides, and rare earth metal ions) from aqueous solution by critically analyzing the most relevant literature studies from the last decade. In the first part of this review, the chemical components of IIPs, the main ion-imprinting technologies as well as the characterization methods used to evaluate the binding properties are briefly presented. In the second part, synthesis parameters, adsorption performance, and a descriptive analysis of solid phase extraction of heavy metal ions by various IIPs are provided.
Keywords: adsorption; heavy metal ions; ion-imprinted polymers; selectivity; wastewater treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures












References
-
- Hu H. Human health and heavy metals exposure. In: McCally M., editor. Life Support: The Environment and Human Health. MIT Press; Cambridge, UK: 2002. pp. 1–12.
-
- Saravan A., Senthil Kumar P., Jeevanantham S., Karishma S., Tajsabreen B., Yaashikaa P.R., Reshma B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere. 2021;280:130595. doi: 10.1016/j.chemosphere.2021.130595. - DOI - PubMed
-
- Ahmed S.F., Mofijur M., Nuzhat S., Tasnim Chowdhury A., Rafa N., Uddin A., Inayat A., Mahlia T.M.I., Ong H.C., Chia W.Y., et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021;416:125912. doi: 10.1016/j.jhazmat.2021.125912. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

