Expanding the Library of 1,2,4-Oxadiazole Derivatives: Discovery of New Farnesoid X Receptor (FXR) Antagonists/Pregnane X Receptor (PXR) Agonists
- PMID: 36985811
- PMCID: PMC10057480
- DOI: 10.3390/molecules28062840
Expanding the Library of 1,2,4-Oxadiazole Derivatives: Discovery of New Farnesoid X Receptor (FXR) Antagonists/Pregnane X Receptor (PXR) Agonists
Abstract
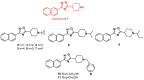
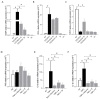
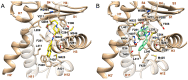
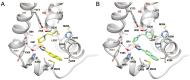
Compounds featuring a 1,2,4-oxadiazole core have been recently identified as a new chemotype of farnesoid X receptor (FXR) antagonists. With the aim to expand this class of compounds and to understand the building blocks necessary to maintain the antagonistic activity, we describe herein the synthesis, the pharmacological evaluation, and the in vitro pharmacokinetic properties of a novel series of 1,2,4-oxadiazole derivatives decorated on the nitrogen of the piperidine ring with different N-alkyl and N-aryl side chains. In vitro pharmacological evaluation showed compounds 5 and 11 as the first examples of nonsteroidal dual FXR/Pregnane X receptor (PXR) modulators. In HepG2 cells, these compounds modulated PXR- and FXR-regulated genes, resulting in interesting leads in the treatment of inflammatory disorders. Moreover, molecular docking studies supported the experimental results, disclosing the ligand binding mode and allowing rationalization of the activities of compounds 5 and 11.
Keywords: 1,2,4-oxadiazole; farnesoid X receptor antagonist; inflammatory disorders; pregnane X receptor agonist.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
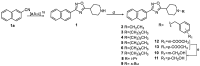
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

