Phytocannabinoids: Chromatographic Screening of Cannabinoids and Loading into Lipid Nanoparticles
- PMID: 36985847
- PMCID: PMC10058297
- DOI: 10.3390/molecules28062875
Phytocannabinoids: Chromatographic Screening of Cannabinoids and Loading into Lipid Nanoparticles
Abstract
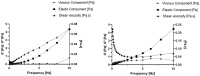
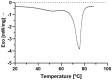
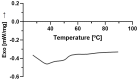
Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) are receiving increasing interest as an approach to encapsulate natural extracts to increase the physicochemical stability of bioactives. Cannabis extract-derived cannabidiol (CBD) has potent therapeutic properties, including anti-inflammatory, antioxidant, and neuroprotective properties. In this work, physicochemical characterization was carried out after producing Compritol-based nanoparticles (cSLN or cNLC) loaded with CBD. Then, the determination of the encapsulation efficiency (EE), loading capacity (LC), particle size (Z-Ave), polydispersity index (PDI), and zeta potential were performed. Additionally, the viscoelastic profiles and differential scanning calorimetry (DSC) patterns were recorded. As a result, CBD-loaded SLN showed a mean particle size of 217.2 ± 6.5 nm, PDI of 0.273 ± 0.023, and EE of about 74%, while CBD-loaded NLC showed Z-Ave of 158.3 ± 6.6 nm, PDI of 0.325 ± 0.016, and EE of about 70%. The rheological analysis showed that the loss modulus for both lipid nanoparticle formulations was higher than the storage modulus over the applied frequency range of 10 Hz, demonstrating that they are more elastic than viscous. The crystallinity profiles of both CBD-cSLN (90.41%) and CBD-cNLC (40.18%) were determined. It may justify the obtained encapsulation parameters while corroborating the liquid-like character demonstrated in the rheological analysis. Scanning electron microscopy (SEM) study confirmed the morphology and shape of the developed nanoparticles. The work has proven that the solid nature and morphology of cSLN/cNLC strengthen these particles' potential to modify the CBD delivery profile for several biomedical applications.
Keywords: Compritol® 888 ATO; Miglyol® 812; cannabidiol; nanostructured lipid carriers; solid lipid nanoparticles; viscoelastic behavior.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
- START 2021/Foundation for Polish Science (FNP)
- 2020.04729.BD/Fundação para a Ciência e a Tecnologia (FCT)
- INNOMED/I/11/NCBR/2014/National Centre for Research and Development
- 2020/04/X/ST5/00789/Institute of Human Genetics, Polish Academy of Sciences by an internal grant for the implementation of a single scientific activity by the National Science Centre within the MINIATURA 4 for single research activity
LinkOut - more resources
Full Text Sources
Research Materials

