Carbon Materials as a Conductive Skeleton for Supercapacitor Electrode Applications: A Review
- PMID: 36985942
- PMCID: PMC10057628
- DOI: 10.3390/nano13061049
Carbon Materials as a Conductive Skeleton for Supercapacitor Electrode Applications: A Review
Abstract
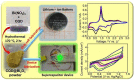
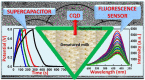
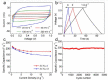
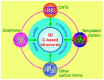
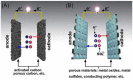
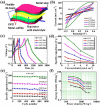
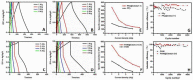
Supercapacitors have become a popular form of energy-storage device in the current energy and environmental landscape, and their performance is heavily reliant on the electrode materials used. Carbon-based electrodes are highly desirable due to their low cost and their abundance in various forms, as well as their ability to easily alter conductivity and surface area. Many studies have been conducted to enhance the performance of carbon-based supercapacitors by utilizing various carbon compounds, including pure carbon nanotubes and multistage carbon nanostructures as electrodes. These studies have examined the characteristics and potential applications of numerous pure carbon nanostructures and scrutinized the use of a wide variety of carbon nanomaterials, such as AC, CNTs, GR, CNCs, and others, to improve capacitance. Ultimately, this study provides a roadmap for producing high-quality supercapacitors using carbon-based electrodes.
Keywords: carbon materials; energy storage; nanoarchitectures; supercapacitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















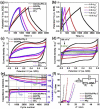
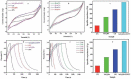

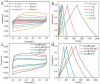


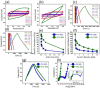


References
-
- Zhong C., Deng Y., Hu W., Sun D., Han X., Qiao J., Zhang J. Electrolytes for Electrochemical Supercapacitors. CRC Press; Boca Raton, FL, USA: 2016. [(accessed on 25 January 2023)]. Available online: https://play.google.com/store/books/details?id=Wu0bDAAAQBAJ.
-
- Sharma K., Arora A., Tripathi S., Storage J.E. Review of supercapacitors: Materials and devices. J. Energy Storage. 2019;21:801–825. doi: 10.1016/j.est.2019.01.010. - DOI
-
- Jiang J., Zhang Y., Nie P., Xu G., Shi M., Wang J., Wu Y., Fu R., Dou H., Zhang X. Progress of nanostructured electrode materials for supercapacitors. [(accessed on 20 January 2023)];Adv. Sustain. Syst. 2018 2:1700110. doi: 10.1002/adsu.201700110. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/adsu.201700110. - DOI - DOI
-
- Yoon J.H., Kumar Y.A., Sambasivam S., Hira S.A., Krishna T.N.V., Zeb K., Uddin W., Kumar K.D., Obaidat I.M., Kim S., et al. Highly efficient copper-cobalt sulfide nano-reeds array with simplistic fabrication strategy for battery-type supercapacitors. J. Energy Storage. 2020;32:101988. doi: 10.1016/j.est.2020.101988. - DOI
-
- Li W., Zeng M., Shao Y., Fan X. Advanced Materials for Supercapacitors. [(accessed on 13 July 2020)];Front. Media SA. 2020 Available online: https://play.google.com/store/books/details?id=v4XzDwAAQBAJ.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

