Magnesium Improves Cardiac Function in Experimental Uremia by Altering Cardiac Elastin Protein Content
- PMID: 36986034
- PMCID: PMC10056411
- DOI: 10.3390/nu15061303
Magnesium Improves Cardiac Function in Experimental Uremia by Altering Cardiac Elastin Protein Content
Abstract
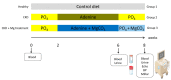
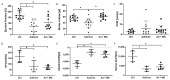
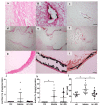
Cardiovascular complications are accompanied by life-threatening complications and represent the major cause of death in patients with chronic kidney disease (CKD). Magnesium is important for the physiology of cardiac function, and its deficiency is common in CKD. In the present study, we investigated the impact of oral magnesium carbonate supplementation on cardiac function in an experimental model of CKD induced in Wistar rats by an adenine diet. Echocardiographic analyses revealed restoration of impaired left ventricular cardiac function in animals with CKD. Cardiac histology and real-time PCR confirmed a high amount of elastin protein and increased collagen III expression in CKD rats supplemented with dietary magnesium as compared with CKD controls. Both structural proteins are crucial in maintaining cardiac health and physiology. Aortic calcium content increased in CKD as compared with tissue from control animals. Magnesium supplementation numerically lowered the increases in aortic calcium content as it remained statistically unchanged, compared with controls. In summary, the present study provides evidence for an improvement in cardiovascular function and aortic wall integrity in a rat model of CKD by magnesium, as evidenced by echocardiography and histology.
Keywords: adenine nephropathy; cardiac function; chronic kidney disease; echocardiography; magnesium.
Conflict of interest statement
S.S. in an employee of Fresenius Medical Care. Parts of this project have been funded by Fresenius Medical Care.
Figures


References
-
- Bouida W., Beltaief K., Msolli M.A., Azaiez N., Ben Soltane H., Sekma A., Trabelsi I., Boubaker H., Grissa M.H., Methemem M., et al. Low-dose Magnesium Sulfate Versus High Dose in the Early Management of Rapid Atrial Fibrillation: Randomized Controlled Double-blind Study (LOMAGHI Study) Acad. Emerg. Med. 2019;26:183–191. doi: 10.1111/acem.13522. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

