Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial
- PMID: 36986131
- PMCID: PMC10055625
- DOI: 10.3390/nu15061402
Early Probiotic Supplementation of Healthy Term Infants with Bifidobacterium longum subsp. infantis M-63 Is Safe and Leads to the Development of Bifidobacterium-Predominant Gut Microbiota: A Double-Blind, Placebo-Controlled Trial
Abstract
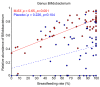
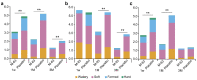
Bifidobacteria are important intestinal bacteria that provide a variety of health benefits in infants. We investigated the efficacy and safety of Bifidobacterium longum subsp. infantis (B. infantis) M-63 in healthy infants in a double-blind, randomized, placebo-controlled trial. Healthy term infants were given B. infantis M-63 (n = 56; 1 × 109 CFU/day) or placebo (n = 54) from postnatal age ≤ 7 days to 3 months. Fecal samples were collected, and fecal microbiota, stool pH, short-chain fatty acids, and immune substances were analyzed. Supplementation with B. infantis M-63 significantly increased the relative abundance of Bifidobacterium compared with the placebo group, with a positive correlation with the frequency of breastfeeding. Supplementation with B. infantis M-63 led to decreased stool pH and increased levels of acetic acid and IgA in the stool at 1 month of age compared with the placebo group. There was a decreased frequency of defecation and watery stools in the probiotic group. No adverse events related to test foods were observed. These results indicate that early supplementation with B. infantis M-63 is well tolerated and contributes to the development of Bifidobacterium-predominant gut microbiota during a critical developmental phase in term infants.
Keywords: Bifidobacterium longum subsp. Infantis; GI motility; gut microbiota; probiotics; secretory IgA; short-chain fatty acids; term infant.
Conflict of interest statement
A.H., M.M., C.X., N.M., S.A., T.O., N.I. and M.T. are employed by Morinaga Milk Industry, Co., Ltd. S.N., T.T. and M.N. have no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

