In Vitro Evaluation of Bioavailability of Se from Daily Food Rations and Dietary Supplements
- PMID: 36986241
- PMCID: PMC10058741
- DOI: 10.3390/nu15061511
In Vitro Evaluation of Bioavailability of Se from Daily Food Rations and Dietary Supplements
Abstract
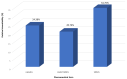
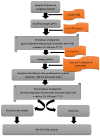
Bioavailability refers to a fraction of a substance that is potentially absorbed from the gastrointestinal tract and enters the systemic circulation (blood). This term is related to various substances, including minerals, that are present in a complex matrix of food which is consumed every day as natural products and pharmaceutical preparations, e.g., dietary supplements. The purpose of the study was to assess the bioavailability of Se from selected dietary supplements, with the simultaneous assessment of the effect the diet type (standard, basic and high-residue diets) has on relative bioavailability. The research included a two-stage in vitro model of digestion using cellulose dialysis tubes of the food rations with the addition of dietary supplements. Se was determined using the ICP-OES method. The bioavailability of Se from dietary supplements, in the presence of food matrix, was determined to be within the range of 19.31-66.10%. Sodium selenate was characterized by the highest value of this parameter, followed by organic forms and sodium selenite. The basic diet, characterized by moderate protein and high carbohydrate and fiber contents, positively influenced the bioavailability of Se. The bioavailability of Se was also influenced by the pharmaceutical form of the product-the highest was for tablets, followed by capsules and coated tablets.
Keywords: ICP-OES; bioavailability; diets; selenium; trace elements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources