Mast Cells in Regeneration of the Skin in Burn Wound with Special Emphasis on Molecular Hydrogen Effect
- PMID: 36986447
- PMCID: PMC10059032
- DOI: 10.3390/ph16030348
Mast Cells in Regeneration of the Skin in Burn Wound with Special Emphasis on Molecular Hydrogen Effect
Abstract
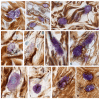
The mechanisms of regeneration for the fibrous component of the connective tissue of the dermis are still insufficiently studied. The aim of this study was to evaluate the effectiveness of the use of molecular hydrogen on the local therapy of a II degree burn wound with the intensification of collagen fibrillogenesis in the skin. We analyzed the involvement of mast cells (MCs) in the regeneration of the collagen fibers of the connective tissue using water with a high content of molecular hydrogen and in a therapeutic ointment for the cell wounds. Thermal burns led to an increase in the skin MC population, accompanied by a systemic rearrangement of the extracellular matrix. The use of molecular hydrogen for the treatment of burn wounds stimulated the regeneration processes by activating the formation of the fibrous component of the dermis, accelerating wound healing. Thus, the intensification of collagen fibrillogenesis was comparable to the effects of a therapeutic ointment. The remodeling of the extracellular matrix correlated with a decrease in the area of damaged skin. Skin regeneration induced by the activation of the secretory activity of MCs may be one of the possible points of implementation of the biological effects of molecular hydrogen in the treatment of burn wounds. Thus, the positive effects of molecular hydrogen on skin repair can be used in clinical practice to increase the effectiveness of therapy after thermal exposure.
Keywords: burn wound; fibrous extracellular matrix; mast cells; molecular hydrogen; regeneration; skin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources

