Development and Stability of a New Formulation of Pentobarbital Suppositories for Paediatric Procedural Sedation
- PMID: 36986615
- PMCID: PMC10055724
- DOI: 10.3390/pharmaceutics15030755
Development and Stability of a New Formulation of Pentobarbital Suppositories for Paediatric Procedural Sedation
Abstract
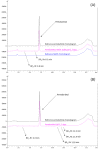
Pentobarbital is a drug of choice to limit motion in children during paediatric procedural sedations (PPSs). However, despite the rectal route being preferred for infants and children, no pentobarbital suppositories are marketed, and therefore they must be prepared by compounding pharmacies. In this study, two suppository formulations of 30, 40, 50, and 60 mg of pentobarbital sodium were developed using hard-fat Witepsol® W25 either alone (formulation F1) or with oleic acid (formulation F2). The two formulations were subjected to the following tests described in the European Pharmacopoeia: uniformity of dosage units, softening time, resistance to rupture, and disintegration time. The stability of both formulations was also investigated for 41 weeks of storage at 5 ± 3 °C using a stability-indicating liquid chromatography method to quantify pentobarbital sodium and research breakdown product (BP). Although both formulae were compliant to uniformity of dosage, the results were in favour of a faster disintegration of F2 compared to F1 (-63%). On the other hand, F1 was found to be stable after 41 weeks of storage unlike F2 for which several new peaks were detected during the chromatographic analysis, suggesting a shorter stability of only 28 weeks. Both formulae still need to be clinically investigated to confirm their safety and efficiency for PPS.
Keywords: formulation; pentobarbital; sedation; stability; suppositories.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

