Application of Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung and Trachea Exposure of Pyronaridine and Artesunate in Hamsters
- PMID: 36986698
- PMCID: PMC10058671
- DOI: 10.3390/pharmaceutics15030838
Application of Minimal Physiologically-Based Pharmacokinetic Model to Simulate Lung and Trachea Exposure of Pyronaridine and Artesunate in Hamsters
Abstract
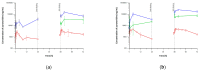
A fixed-dose combination of pyronaridine and artesunate, one of the artemisinin-based combination therapies, has been used as a potent antimalarial treatment regimen. Recently, several studies have reported the antiviral effects of both drugs against severe acute respiratory syndrome coronavirus two (SARS-CoV-2). However, there are limited data on the pharmacokinetics (PKs), lung, and trachea exposures that could be correlated with the antiviral effects of pyronaridine and artesunate. The purpose of this study was to evaluate the pharmacokinetics, lung, and trachea distribution of pyronaridine, artesunate, and dihydroartemisinin (an active metabolite of artesunate) using a minimal physiologically-based pharmacokinetic (PBPK) model. The major target tissues for evaluating dose metrics are blood, lung, and trachea, and the nontarget tissues were lumped together into the rest of the body. The predictive performance of the minimal PBPK model was evaluated using visual inspection between observations and model predictions, (average) fold error, and sensitivity analysis. The developed PBPK models were applied for the multiple-dosing simulation of daily oral pyronaridine and artesunate. A steady state was reached about three to four days after the first dosing of pyronaridine and an accumulation ratio was calculated to be 1.8. However, the accumulation ratio of artesunate and dihydroartemisinin could not be calculated since the steady state of both compounds was not achieved by daily multiple dosing. The elimination half-life of pyronaridine and artesunate was estimated to be 19.8 and 0.4 h, respectively. Pyronaridine was extensively distributed to the lung and trachea with the lung-to-blood and trachea-to-blood concentration ratios (=Cavg,tissue/Cavg,blood) of 25.83 and 12.41 at the steady state, respectively. Also, the lung-to-blood and trachea-to-blood AUC ratios for artesunate (dihydroartemisinin) were calculated to be 3.34 (1.51) and 0.34 (0.15). The results of this study could provide a scientific basis for interpreting the dose-exposure-response relationship of pyronaridine and artesunate for COVID-19 drug repurposing.
Keywords: COVID-19; artesunate; drug repurposing; minimal physiologically-based pharmacokinetic (PBPK) model; pyronaridine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Chakraborty C., Sharma A.R., Bhattacharya M., Agoramoorthy G., Lee S.-S. The drug repurposing for COVID-19 clinical trials provide very effective therapeutic combinations: Lessons learned from major clinical studies. Front. Pharmacol. 2021;12:2942. doi: 10.3389/fphar.2021.704205. - DOI - PMC - PubMed
-
- Puhl A.C., Fritch E.J., Lane T.R., Tse L.V., Yount B.L., Sacramento C.Q., Fintelman-Rodrigues N., Tavella T.A., Maranhão Costa F.T., Weston S. Repurposing the ebola and marburg virus inhibitors tilorone, quinacrine, and pyronaridine: In vitro activity against SARS-CoV-2 and potential mechanisms. ACS Omega. 2021;6:7454–7468. doi: 10.1021/acsomega.0c05996. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

