Radiolabeled Risperidone microSPECT/CT Imaging for Intranasal Implant Studies Development
- PMID: 36986704
- PMCID: PMC10054269
- DOI: 10.3390/pharmaceutics15030843
Radiolabeled Risperidone microSPECT/CT Imaging for Intranasal Implant Studies Development
Abstract
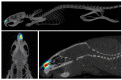
The use of intranasal implantable drug delivery systems has many potential advantages for the treatment of different diseases, as they can provide sustained drug delivery, improving patient compliance. We describe a novel proof-of-concept methodological study using intranasal implants with radiolabeled risperidone (RISP) as a model molecule. This novel approach could provide very valuable data for the design and optimization of intranasal implants for sustained drug delivery. RISP was radiolabeled with 125I by solid supported direct halogen electrophilic substitution and added to a poly(lactide-co-glycolide) (PLGA; 75/25 D,L-Lactide/glycolide ratio) solution that was casted on top of 3D-printed silicone molds adapted for intranasal administration to laboratory animals. Implants were intranasally administered to rats, and radiolabeled RISP release followed for 4 weeks by in vivo non-invasive quantitative microSPECT/CT imaging. Percentage release data were compared with in vitro ones using radiolabeled implants containing either 125I-RISP or [125I]INa and also by HPLC measurement of drug release. Implants remained in the nasal cavity for up to a month and were slowly and steadily dissolved. All methods showed a fast release of the lipophilic drug in the first days with a steadier increase to reach a plateau after approximately 5 days. The release of [125I]I- took place at a much slower rate. We herein demonstrate the feasibility of this experimental approach to obtain high-resolution, non-invasive quantitative images of the release of the radiolabeled drug, providing valuable information for improved pharmaceutical development of intranasal implants.
Keywords: SPECT/CT; intranasal implant; molecular imaging; radioiodination; risperidone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Li W., Tang J., Lee D., Tice T.R., Schwendeman S.P., Prausnitz M.R. Clinical translation of long-acting drug delivery formulations. Nat. Rev. Mater. 2022;7:406–420. doi: 10.1038/s41578-021-00405-w. - DOI
-
- Chappel E. Drug Delivery Devices and Therapeutic Systems. Elsevier; Amsterdam, The Netherlands: 2021. Implantable drug delivery devices; pp. 129–156.
-
- Korelidou A., Domínguez-Robles J., Magill E., Eleftheriadou M., Cornelius V.A., Donnelly R.F., Margariti A., Larrañeta E. 3D-printed reservoir-type implants containing poly(lactic acid)/poly(caprolactone) porous membranes for sustained drug delivery. Biomater. Adv. 2022;139:213024. doi: 10.1016/j.bioadv.2022.213024. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

