Evaluation of Pharmacobezoar Formation from Suspensions of Spray-Dried Amorphous Solid Dispersions: An MRI Study in Rats
- PMID: 36986751
- PMCID: PMC10052685
- DOI: 10.3390/pharmaceutics15030887
Evaluation of Pharmacobezoar Formation from Suspensions of Spray-Dried Amorphous Solid Dispersions: An MRI Study in Rats
Abstract
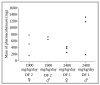
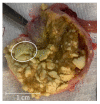
Spray-dried amorphous solid dispersions of new chemical entities and pH-dependent soluble polymer hydroxypropyl methylcellulose acetate succinate (HPMC-AS) were found to form solid agglomerates in the gastrointestinal tract of rodents after oral administration. These agglomerates, referring to descriptions of intra-gastrointestinal aggregated oral dosage forms termed pharmacobezoars, represent a potential risk for animal welfare. Previously, we introduced an in vitro model to assess the agglomeration potential of amorphous solid dispersions from suspensions and how it can be reduced. In this work, we investigated if the in vitro effective approach of viscosity enhancement of the vehicle used to prepare suspensions of amorphous solid dispersions could reduce the pharmacobezoar formation potential following repeated daily oral dosing to rats as well. The dose level of 2400 mg/kg/day used in the main study was determined in a dose finding study carried out in advance. In the dose finding study, MRI investigations were carried out at short time intervals to gain insights into the process of pharmacobezoar formation. Whereas MRI investigations underlined the importance of the forestomach for the formation of pharmacobezoars, viscosity enhancement of the vehicle reduced the incidence of pharmacobezoars, delayed the onset of pharmacobezoar formation and reduced the overall mass of pharmacobezoars found at necropsy.
Keywords: MRI study; pharmacobezoars; preclinical testing; rodent stomach; spray-dried amorphous solid dispersions; viscosity.
Conflict of interest statement
This study was performed in cooperation between the Department of Biopharmaceutics and Pharmaceutical Technology of the University of Greifswald and Boehringer Ingelheim Pharma GmbH & Co. KG. The Article Processing Charge was funded by Boehringer Ingelheim Pharma GmbH & Co. KG. No additional funding was applied.
Figures








References
-
- Archibald K., Coleman R., Drake T. Animal Experimentation: Working towards a Paradigm Change. BRILL; Leiden, The Netherlands: 2019. Replacing animal tests to improve safety for humans; pp. 417–422.
-
- ICH Guideline M3 (R2)—Non-Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorisation for Pharmaceuticals. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human. 2009. [(accessed on 19 May 2022)]. Available online: http://www.ich.org/products/guidelines/multidisciplinary/article/multidi....
LinkOut - more resources
Full Text Sources
Research Materials

