Micro-Injection Moulding of PEO/PCL Blend-Based Matrices for Extended Oral Delivery of Fenbendazole
- PMID: 36986761
- PMCID: PMC10051197
- DOI: 10.3390/pharmaceutics15030900
Micro-Injection Moulding of PEO/PCL Blend-Based Matrices for Extended Oral Delivery of Fenbendazole
Abstract
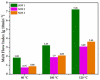
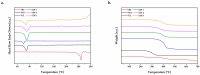
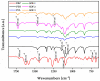
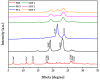
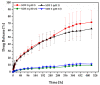
Fenbendazole (FBZ) is a broad-spectrum anthelmintic administered orally to ruminants; nevertheless, its poor water solubility has been the main limitation to reaching satisfactory and sustained levels at the site of the target parasites. Hence, the exploitation of hot-melt extrusion (HME) and micro-injection moulding (µIM) for the manufacturing of extended-release tablets of plasticised solid dispersions of poly(ethylene oxide) (PEO)/polycaprolactone (PCL) and FBZ was investigated due to their unique suitability for semi-continuous manufacturing of pharmaceutical oral solid dosage forms. High-performance liquid chromatography (HPLC) analysis demonstrated a consistent and uniform drug content in the tablets. Thermal analysis using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) suggested the amorphous state of the active ingredient, which was endorsed by powder X-ray diffraction spectroscopy (pXRD). Fourier transform infrared spectroscopy (FTIR) analysis did not display any new peak indicative of either a chemical interaction or degradation. Scanning electron microscopy (SEM) images showed smoother surfaces and broader pores as we increased the PCL content. Electron-dispersive X-ray spectroscopy (EDX) revealed that the drug was homogeneously distributed within the polymeric matrices. Drug release studies attested that all moulded tablets of amorphous solid dispersions improved the drug solubility, with the PEO/PCL blend-based matrices showing drug release by Korsmeyer-Peppas kinetics. Thus, HME coupled with µIM proved to be a promising approach towards a continuous automated manufacturing process for the production of oral solid dispersions of benzimidazole anthelmintics to grazing cattle.
Keywords: animal health; extended-release; fenbendazole; hot-melt extrusion; micro-injection moulding; solid dispersion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Sabnis S., Rathbone M.J. Long Acting Animal Health Drug Products. Advances in Delivery Science and Technology. Springer; New York, NY, USA: 2013. Animal Health Markets and Opportunities: Farmed Animal Landscape; pp. 1–14.
-
- Ellis K.J. Long Acting Animal Health Drug Products. Advances in Delivery Science and Technology. Springer; New York, NY, USA: 2013. Anatomy and Physiology of the Farmed Animal; pp. 47–58.
Grants and funding
LinkOut - more resources
Full Text Sources

