Hospital Production of Sterile 2% Propofol Nanoemulsion: Proof of Concept
- PMID: 36986768
- PMCID: PMC10058537
- DOI: 10.3390/pharmaceutics15030905
Hospital Production of Sterile 2% Propofol Nanoemulsion: Proof of Concept
Abstract
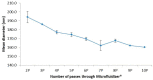
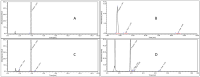
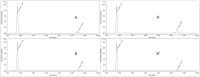
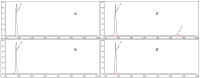
In the context of essential drug shortages, this article reports a proof of concept for the hospital preparation of a 2% propofol injectable nanoemulsion. Two processes for propofol were assessed: mixing propofol with the commercial Intralipid® 20% emulsion and a "de novo" process performed using separate raw materials (i.e., oil, water, and surfactant) and optimized for droplet size reduction with a high-pressure homogenizer. A propofol HPLC-UV stability-indicating method was developed for process validation and short-term stability. In addition, free propofol in the aqueous phase was quantified by dialysis. To envision routine production, sterility and endotoxin tests were validated. Only the "de novo" process using high-pressure homogenization gave satisfactory physical results similar to commercialized Diprivan® 2%. Both terminal heat sterilization processes (121 °C, 15 min and 0.22 µm filtration) were validated, but an additional pH adjustment was required prior to heat sterilization. The propofol nanoemulsion was monodisperse with a 160 nm mean droplet size, and no droplets were larger than 5µm. We confirmed that free propofol in the aqueous phase of the emulsion was similar to Diprivan 2%, and the chemical stability of propofol was validated. In conclusion, the proof of concept for the in-house 2% propofol nanoemulsion preparation was successfully demonstrated, opening the field for the possible production of the nanoemulsion in hospital pharmacies.
Keywords: drug shortages; hospital preparation; nanoemulsion; propofol; sterile.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Norouzi P., Rastegari A., Mottaghitalab F., Farokhi M., Zarrintaj P., Saeb M.R. Nanoemulsions for intravenous drug delivery. Nanoeng. Biomat. Adv. Drug. Del. 2020;24:582–601. doi: 10.1016/B978-0-08-102985-5.00024-3. - DOI
-
- Mcclement D. Nanoemulsions versus microemulsions: Terminology, differences, similarities. Soft Matter. 2012;8:1719–1729. doi: 10.1039/C2SM06903B. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

