Clinically Expired Platelet Concentrates as a Source of Extracellular Vesicles for Targeted Anti-Cancer Drug Delivery
- PMID: 36986815
- PMCID: PMC10056378
- DOI: 10.3390/pharmaceutics15030953
Clinically Expired Platelet Concentrates as a Source of Extracellular Vesicles for Targeted Anti-Cancer Drug Delivery
Abstract
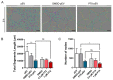
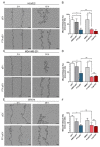
The short shelf life of platelet concentrates (PC) of up to 5-7 days leads to higher wastage due to expiry. To address this massive financial burden on the healthcare system, alternative applications for expired PC have emerged in recent years. Engineered nanocarriers functionalized with platelet membranes have shown excellent targeting abilities for tumor cells owing to their platelet membrane proteins. Nevertheless, synthetic drug delivery strategies have significant drawbacks that platelet-derived extracellular vesicles (pEV) can overcome. We investigated, for the first time, the use of pEV as a carrier of the anti-breast cancer drug paclitaxel, considering it as an appealing alternative to improve the therapeutic potential of expired PC. The pEV released during PC storage showed a typical EV size distribution profile (100-300 nm) with a cup-shaped morphology. Paclitaxel-loaded pEV showed significant anti-cancer effects in vitro, as demonstrated by their anti-migratory (>30%), anti-angiogenic (>30%), and anti-invasive (>70%) properties in distinct cells found in the breast tumor microenvironment. We provide evidence for a novel application for expired PC by suggesting that the field of tumor treatment research may be broadened by the use of natural carriers.
Keywords: anti-angiogenic potential; drug delivery system; expired platelet concentrates; human breast cancer cell line; paclitaxel; platelet-derived extracellular vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- World Health Organization. WHO Model List of Essential Medicines: 20th List. 2017. [(accessed on 2 November 2022)]. Available online: https://apps.who.int/iris/handle/10665/273826.
Grants and funding
- PTDC/BTM-ORG/32187/201; UIDB/04462/2020 and UIDP/04462/2020; 2022.10467.PTDC/Fundação para a Ciência e Tecnologia funded projects PEFPlateletValue; iNOVA4Health; EXCELERATE
- LA/P/0087/2020/FCT/Ministério da Ciência, Tecnologia e 893 Ensino Superior, through national funds;Associate Laboratory LS4FUTURE
LinkOut - more resources
Full Text Sources

