Sinonasal Stent Coated with Sustained-Release Varnish of Mometasone Furoate Inhibits Pro-Inflammatory Cytokine Release from Macrophages: An In Vitro Study
- PMID: 36986875
- PMCID: PMC10051169
- DOI: 10.3390/pharmaceutics15031015
Sinonasal Stent Coated with Sustained-Release Varnish of Mometasone Furoate Inhibits Pro-Inflammatory Cytokine Release from Macrophages: An In Vitro Study
Abstract
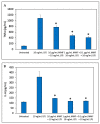
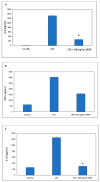
The aim of the study was to develop a sustained-release varnish (SRV) containing mometasone furoate (MMF) for sinonasal stents (SNS) to reduce mucosa inflammation in the sinonasal cavity. The SNS' segments coated with SRV-MMF or an SRV-placebo were incubated daily in a fresh DMEM at 37 °C for 20 days. The immunosuppressive activity of the collected DMEM supernatants was tested on the ability of mouse RAW 264.7 macrophages to secrete the cytokines' tumor necrosis factor α (TNFα) and interleukin (IL)-10 and IL-6 in response to lipopolysaccharide (LPS). The cytokine levels were determined by respective Enzyme-Linked Immunosorbent Assays (ELISAs). We found that the daily amount of MMF released from the coated SNS was sufficient to significantly inhibit LPS-induced IL-6 and IL-10 secretion from the macrophages up to days 14 and 17, respectively. SRV-MMF had, however, only a mild inhibitory effect on LPS-induced TNFα secretion as compared to the SRV-placebo-coated SNS. In conclusion, the coating of SNS with SRV-MMF provides a sustained delivery of MMF for at least 2 weeks, maintaining a level sufficient for inhibiting pro-inflammatory cytokine release. This technological platform is, therefore, expected to provide anti-inflammatory benefits during the postoperative healing period and may play a significant role in the future treatment of chronic rhinosinusitis.
Keywords: chronic rhinosinusitis; cytokine; local drug delivery; nasal stent; steroids; sustained-release varnish.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Polyglactin 910 Meshes Coated with Sustained-Release Cannabigerol Varnish Inhibit Staphylococcus aureus Biofilm Formation and Macrophage Cytokine Secretion: An In Vitro Study.Pharmaceuticals (Basel). 2023 May 13;16(5):745. doi: 10.3390/ph16050745. Pharmaceuticals (Basel). 2023. PMID: 37242528 Free PMC article.
-
Sinonasal Stent Coated with Slow-Release Varnish of Chlorhexidine Has Sustained Protection against Bacterial Biofilm Growth in the Sinonasal Cavity: An In Vitro Study.Pharmaceutics. 2021 Oct 25;13(11):1783. doi: 10.3390/pharmaceutics13111783. Pharmaceutics. 2021. PMID: 34834197 Free PMC article.
-
Sustained release varnish containing chlorhexidine for prevention of Streptococcus mutans biofilm formation on voice prosthesis surface: an in vitro study.Int Microbiol. 2022 Jan;25(1):177-187. doi: 10.1007/s10123-021-00205-w. Epub 2021 Sep 9. Int Microbiol. 2022. PMID: 34505216
-
Prolonged Inhibition of Streptococcus mutans Growth and Biofilm Formation by Sustained Release of Chlorhexidine from Varnish Coated Dental Abutments: An in Vitro Study.Int J Dent. 2022 Oct 14;2022:7246155. doi: 10.1155/2022/7246155. eCollection 2022. Int J Dent. 2022. PMID: 36275203 Free PMC article.
-
Inhaled mometasone furoate: a review of its use in adults and adolescents with persistent asthma.Drugs. 2001;61(9):1325-50. doi: 10.2165/00003495-200161090-00011. Drugs. 2001. PMID: 11511026 Review.
Cited by
-
The Incorporation of CBD into Biodegradable DL-Lactide/Glycolide Copolymers Creates a Persistent Antibacterial Environment: An In Vitro Study on Streptococcus mutans and Staphylococcus aureus.Pharmaceutics. 2025 Apr 2;17(4):463. doi: 10.3390/pharmaceutics17040463. Pharmaceutics. 2025. PMID: 40284458 Free PMC article.
-
Polyglactin 910 Meshes Coated with Sustained-Release Cannabigerol Varnish Inhibit Staphylococcus aureus Biofilm Formation and Macrophage Cytokine Secretion: An In Vitro Study.Pharmaceuticals (Basel). 2023 May 13;16(5):745. doi: 10.3390/ph16050745. Pharmaceuticals (Basel). 2023. PMID: 37242528 Free PMC article.
References
-
- Huang Z., Zhou B., Wang D., Zang H., Zhang H., Wang H., Wang S., Cheng L., Li J., Wu W., et al. Comparison of Bioabsorbable Steroid-Eluting Sinus Stents Versus Nasopore After Endoscopic Sinus Surgery: A Multicenter, Randomized, Controlled, Single-Blinded Clinical Trial. Ear Nose Throat J. 2022;101:260–267. doi: 10.1177/0145561320947632. - DOI - PubMed
LinkOut - more resources
Full Text Sources

