The Protective Effect of Exogenous Ascorbic Acid on Photosystem Inhibition of Tomato Seedlings Induced by Salt Stress
- PMID: 36987066
- PMCID: PMC10052531
- DOI: 10.3390/plants12061379
The Protective Effect of Exogenous Ascorbic Acid on Photosystem Inhibition of Tomato Seedlings Induced by Salt Stress
Abstract
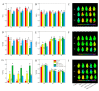
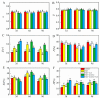
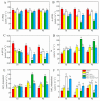
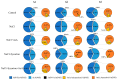
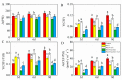
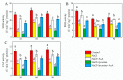
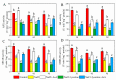
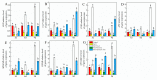
This study investigated the protective effects of exogenous ascorbic acid (AsA, 0.5 mmol·L-1) treatment on salt-induced photosystem inhibition in tomato seedlings under salt stress (NaCl, 100 mmol·L-1) conditions with and without the AsA inhibitor lycorine. Salt stress reduced the activities of photosystem II (PSII) and PSI. AsA treatment mitigated inhibition of the maximal photochemical efficiency of PSII (Fv/Fm), maximal P700 changes (Pm), the effective quantum yields of PSII and I [Y(II) and Y(I)], and non-photochemical quenching coefficient (NPQ) values under salt stress conditions both with and without lycorine. Moreover, AsA restored the balance of excitation energy between two photosystems (β/α-1) after disruption by salt stress, with or without lycorine. Treatment of the leaves of salt-stressed plants with AsA with or without lycorine increased the proportion of electron flux for photosynthetic carbon reduction [Je(PCR)] while decreasing the O2-dependent alternative electron flux [Ja(O2-dependent)]. AsA with or without lycorine further resulted in increases in the quantum yield of cyclic electron flow (CEF) around PSI [Y(CEF)] while increasing the expression of antioxidant and AsA-GSH cycle-related genes and elevating the ratio of reduced glutathione/oxidized glutathione (GSH/GSSG). Similarly, AsA treatment significantly decreased the levels of reactive oxygen species [superoxide anion (O2-) and hydrogen peroxide (H2O2)] in these plants. Together, these data indicate that AsA can alleviate salt-stress-induced inhibition of PSII and PSI in tomato seedlings by restoring the excitation energy balance between the photosystems, regulating the dissipation of excess light energy by CEF and NPQ, increasing photosynthetic electron flux, and enhancing the scavenging of reactive oxygen species, thereby enabling plants to better tolerate salt stress.
Keywords: ascorbic acid; photoprotection; photosystem inhibition; salt stress; tomato.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Hossain M.S., Alam M.U., Rahman A., Hasanuzzaman M., Nahar K., Mahmud J.A., Fujita M. Use of iso-osmotic solution to understand salt stress responses in lentil (Lens culinaris Medik.) S. Afr. J. Bot. 2017;113:346–354. doi: 10.1016/j.sajb.2017.09.007. - DOI
-
- Mushtaq Z., Faizan S., Gulzar B. Salt stress, its impacts on plants and the strategies plants are employing against it: A review. J. Appl. Biol. Biotechnol. 2020;8:81–91. doi: 10.7324/JABB.2020.80315. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

