A Hydrocarbon Soluble, Molecular and "Complete" Al-Cocatalyst for High Temperature Olefin Polymerization
- PMID: 36987158
- PMCID: PMC10051415
- DOI: 10.3390/polym15061378
A Hydrocarbon Soluble, Molecular and "Complete" Al-Cocatalyst for High Temperature Olefin Polymerization
Abstract
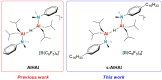
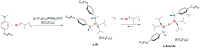
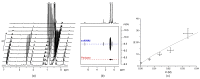
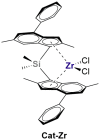
The dinuclear aluminum salt {[iBu2(DMA)Al]2(μ-H)}+[B(C6F5)4]- (AlHAl; DMA = N,N-dimethylaniline) is the prototype of a new class of molecular cocatalysts for catalytic olefin polymerization, its modular nature offering easy avenues for tailoring the activator to specific needs. We report here, as proof of concept, a first variant (s-AlHAl) bearing p-hexadecyl-N,N-dimethylaniline (DMAC16) units, which enhances solubility in aliphatic hydrocarbons. The novel s-AlHAl was used successfully as an activator/scavenger in ethylene/1-hexene copolymerization in a high-temperature solution process.
Keywords: borate activators; catalyst activation; methylaluminoxane; olefin polymerization; soluble cocatalyst.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Zaccaria F., Sian L., Zuccaccia C., Macchioni A. Ion Pairing in Transition Metal Catalyzed Olefin Polymerization. In: Perez P.J., editor. Advances in Organometallic Chemistry. Volume 73. Elsevier; Amsterdam, The Netherlands: 2020. pp. 1–78.
-
- Wild F.R.W.P., Zsolnai L., Huttner G., Brintzinger H.H. Ansa-Metallocene Derivatives. IV. Synthesis and Molecular Structures of Chiral Ansa-Titanocene Derivatives with Bridged Tetrahydroindenyl Ligands. J. Organomet. Chem. 1982;232:233–247. doi: 10.1016/S0022-328X(82)80018-1. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

