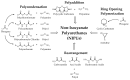
Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs)
- PMID: 36987369
- PMCID: PMC10051735
- DOI: 10.3390/polym15061589
Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs)
Abstract
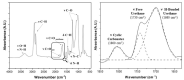
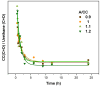
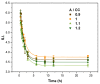
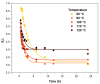
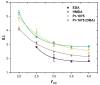
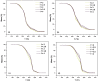
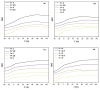
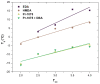
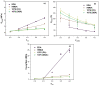
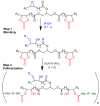
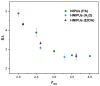
Non-isocyanate polyurethanes (NIPUs) based on biobased polyamines and polycarbonates are a sustainable alternative to conventional polyurethanes (PU). This article discloses a novel method to control the crosslinking density of fully biobased isocyanate-free polyurethanes, synthesized from triglycerides carbonated previously in scCO2 and different diamines, such as ethylenediamine (EDA), hexamethylenediamine (HMDA) and PriamineTM-1075 (derived from a dimerized fatty acid). As capping substances, water or bioalcohols are used in such a way that the crosslinking density can be adjusted to suit the requirements of the intended application. An optimization of the NIPU synthesis procedure is firstly carried out, establishing the polymerization kinetics and proposing optimal conditions set for the synthesis of the NIPUs. Then, the influence of the partial blocking of the active polymerization sites of the carbonated soybean oil (CSBO), using monofunctional amines, on the physical properties of the NIPUS is explored. Finally, the synthesis of fully biobased NIPUs with a targeted crosslinking density is achieved using hybrid NIPUs, employing partially carbonated oil and H2O or ethanol as blockers to achieve the desired physical properties in a very precise manner.
Keywords: CSBO; NIPU; crosslink; cyclic carbonate; priamine; tailor-made; vegetable oil.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Polyurethane Market Size, Share & Trends Analysis Report by Product (Flexible Foam, Rigid Foam), by End Use (Construction, Electronics & Appliances), by Region (APAC, North America), and Segment Forecasts, 2021–2028, (n.d.) [(accessed on 8 April 2022)]. Available online: https://www.researchandmarkets.com/reports/4118824/polyurethane-market-s....
-
- Szycher M. Szycher’s Handbook of Polyurethanes. 2nd ed. Taylor & Francis; Abingdon, UK: 2013. - DOI
-
- Xie F., Zhang T., Bryant P., Kurusingal V., Colwell J.M., Laycock B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019;90:211–268. doi: 10.1016/j.progpolymsci.2018.12.003. - DOI
-
- Gunatillake P.A., Martin D.J., Meijs G.F., McCarthy S.J., Adhikari R. Designing biostable polyurethane elastomers for biomedical implants. Aust. J. Chem. 2003;56:545–557. doi: 10.1071/CH02168. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

