Intestinal and optic-cup organoids as tools for unveiling mechanics of self-organizing morphogenesis
- PMID: 36987402
- PMCID: PMC10040261
- DOI: 10.2142/biophysico.bppb-v19.0048
Intestinal and optic-cup organoids as tools for unveiling mechanics of self-organizing morphogenesis
Abstract
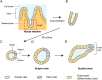
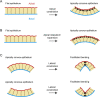
Organoid, an organ-like tissue reproduced in a dish, has specialized, functional structures in three-dimensional (3D) space. Organoid development replicates the self-organizing process of each tissue development during embryogenesis but does not necessarily require external tissues, illustrating the autonomy of multicellular systems. Herein, we review the developmental processes of epithelial organoids, namely, the intestine, and optic-cup, with a focus on their mechanical aspects. Recent organoid studies have advanced our understanding of the mechanisms of 3D tissue deformation, including appropriate modes of deformation and factors controlling them. In addition, the autonomous nature of organoid development has also allowed us to access the stepwise mechanisms of deformation as organoids proceed through distinct stages of development. Altogether, we discuss the potential of organoids in unveiling the autonomy of multicellular self-organization from a mechanical point of view. This review article is an extended version of the Japanese article, Mechanics in Self-organizing Organoid Morphogenesis, published in SEIBUTSU BUTSURI Vol. 60, p.31-36 (2020).
Keywords: cell autonomy; development; intestinal organoid; optic-cup organoid; tissue mechanics.
2022 THE BIOPHYSICAL SOCIETY OF JAPAN.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Eiraku, M., Watanabe, K., Matsuo-Takasaki, M., Kawada, M., Yonemura, S., Matsumura, M., et al. . Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008). https://doi.org/10.1016/j.stem.2008.09.002 - PubMed
-
- Sato, T., Vries, R. G., Snippert, H. J., van de Wetering, M., Barker, N., Stange, D. E., et al. . Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009). http://dx.doi.org/10.1038/nature07935 - PubMed
-
- Spence, J. R., Mayhew, C. N., Rankin, S. A., Kuhar, M. F., Vallance, J. E., Tolle, K., et al. . Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011). http://dx.doi.org/10.1038/nature09691 - PMC - PubMed
-
- Eiraku, M., Takata, N., Ishibashi, H., Kawada, M., Sakakura, E., Okuda, S., et al. . Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011). http://dx.doi.org/10.1038/nature09941 - PubMed
-
- Lancaster, M. A., Knoblich, J. A.. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 345, 1247125 (2014). https://doi.org/10.1126/science.1247125 - PubMed

